SNP (Single Nucleotide Polymorphism) marker, primer and kit for evaluating solid organ transplantation condition and use method of SNP marker, primer and kit
A solid and state-of-the-art technology, applied in the field of SNP markers to evaluate the state of solid organ transplantation, can solve the problems of influence on the accuracy of results, low accuracy and sensitivity, and achieve reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Using the kit provided by the invention to assess the risk of graft injury and rejection after solid organ transplantation
[0135] Take kidney transplantation testing as an example:
[0136] 1. Collect oral epithelial cells from kidney transplant recipients and donors respectively, extract DNA, and adjust the concentration to 10-40ng / ul
[0137] 2. Configure the SNP screening reaction system separately and perform PCR reaction; the reaction preparation is as follows:
[0138] Table 2
[0139]
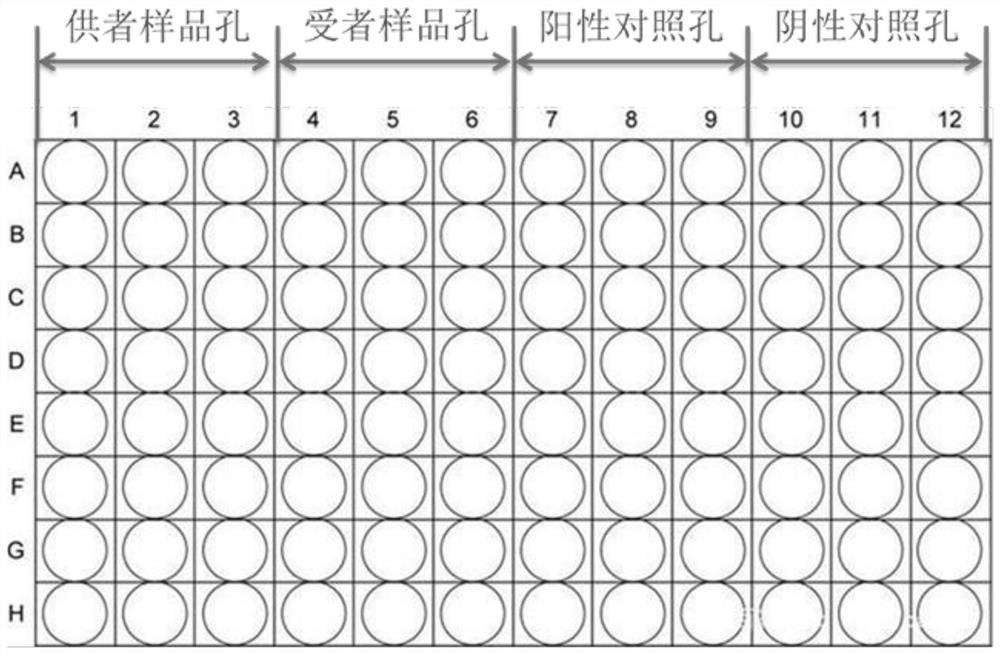
[0140] 3. Add the configured reaction system to the 96-well plate for SNP screening as required, see figure 1 , (the 96-well plate includes: donor sample wells, recipient sample wells, negative control wells, and positive control wells arranged in sequence from left to right; donor sample wells, recipient sample wells, negative control wells, and positive control wells are all 3 columns and 8 rows)
[0141] 4. Then use the aluminum sealing film to cooperate with the seali...
Embodiment 2
[0167] Effect data:
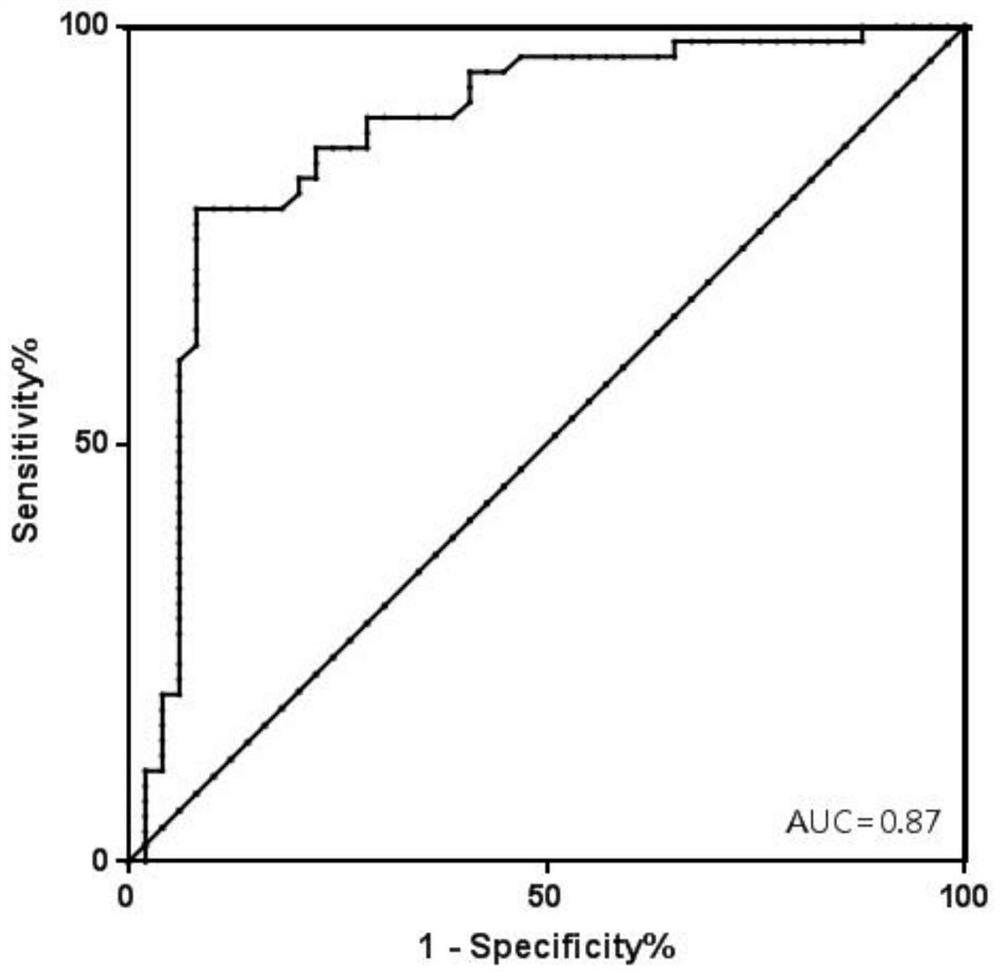
[0168] Include 742 recipients after kidney transplantation, collect their plasma according to the method in Example 1, extract the total free DNA in the plasma, and perform digital PCR reaction. After the reaction, analyze each SNP site to obtain the percentage of GcfDNA, set 1 % is the reference value to distinguish graft injury or rejection from normal (graft injury or rejection (GcfDNA percentage > 1%) and graft normal group (GcfDNA percentage < 1%)).
[0169] Use MedCalc software to analyze, obtain the ROC curve ( figure 2 ), AUC=0.87, sensitivity=81.82%, specificity=79.59%.
[0170] The kit of the invention can timely, accurately and specifically reflect the health status of the kidney graft, and evaluate the graft injury and rejection risk after liver transplantation.
Embodiment 3
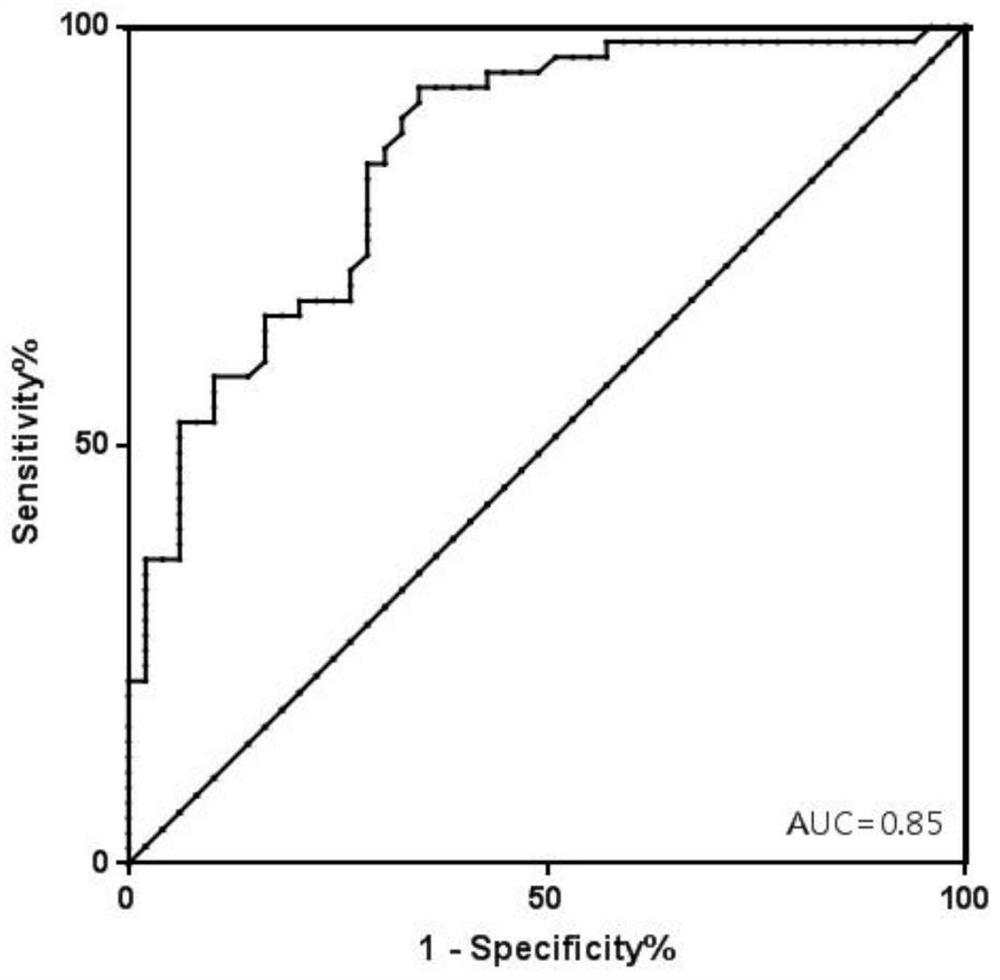
[0172] Include 416 recipients after liver transplantation, collect their plasma according to Example 1, extract the total free DNA in the plasma, and perform digital PCR reaction. After the reaction, analyze each SNP site to obtain the percentage of GcfDNA, set 10% Graft injury or rejection was distinguished from normal for reference values (graft injury or rejection (GcfDNA percentage > 10%) and graft normal group (GcfDNA percentage < 10%)).
[0173]Effect data: use MedCalc software to analyze, obtain the ROC curve ( image 3 ), AUC=0.85, sensitivity=83.64%, specificity=71.43%.
[0174] The kit of the invention can timely, accurately and specifically monitor the health status of the liver graft, and evaluate the graft injury and rejection risk after liver transplantation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com