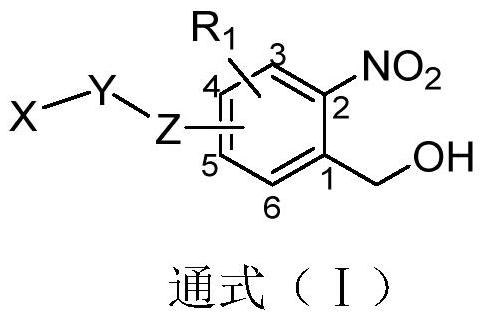
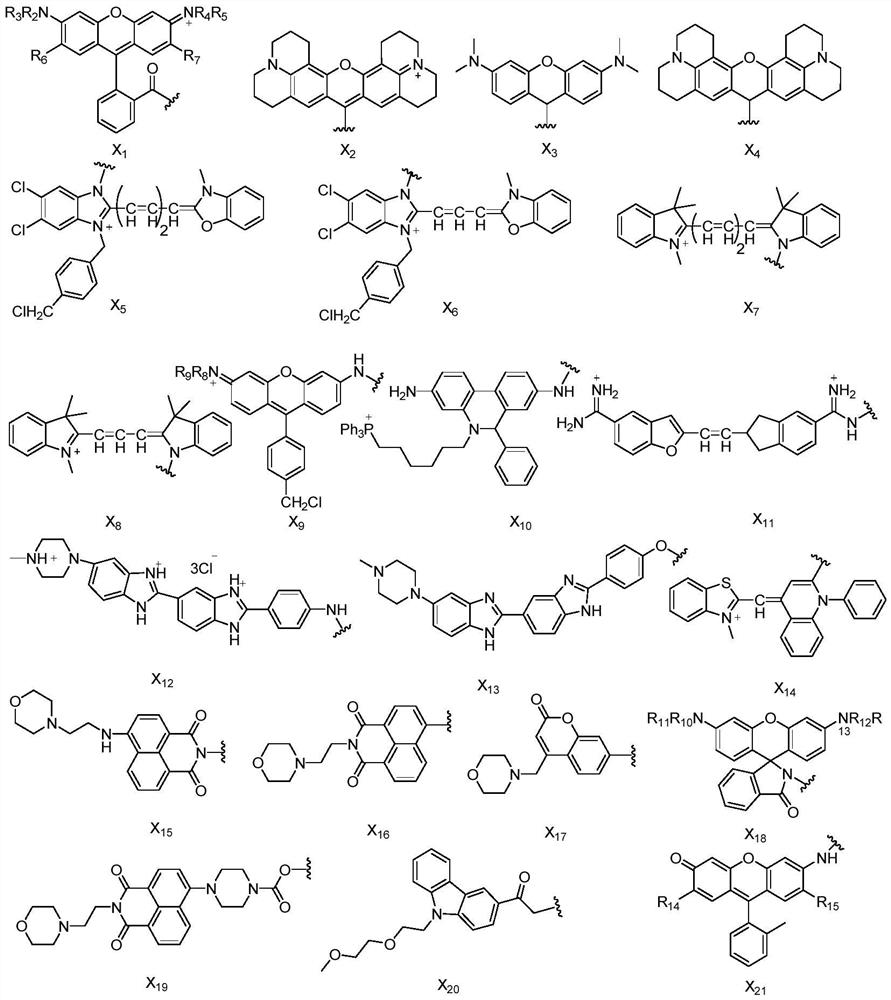
Photo-induced cell covalent labeled fluorescent molecules as well as preparation method and application thereof
A compound, selected technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem that the chemical reaction functional group has not been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0126] Example 3: 4-(Hydroxymethyl)-N-(3-(4-(5-(4-methylpiperazin-1-yl)-1H,1'H-[2,5'-biphenyl And [d] imidazolium] -2'-yl) phenoxy) propyl) -3-nitrobenzamide (3)
[0127]
[0128] Step 3-1: Compound 3-A (10mg, 0.01873mmol) was dissolved in 1mL DMF, potassium carbonate (13mg, 0.09365mmol) and tert-butyl (3-bromopropyl)carbamate (6.7mg, 0.0282mmol) were added, Reaction overnight at 50°C, spin-dry column chromatography directly after the reaction, the product is a yellow-green solid (10.1mg, 92%).ESI-MS[M-H] - m / z=580.47.[M+H] + m / z=582.46. 1 H NMR (600MHz, DMSO) δ12.55(s, 1H), 8.23(m, 3H), 8.14(d, J=8.7Hz, 2H), 8.00(d, J=8.4Hz, 1H), 7.66(m ,1H),7.44(m,1H),7.12(d,J=8.8Hz,2H),6.94(d,J=8.5Hz,2H),4.08(t,J=6.2Hz,2H),3.11(dd ,J=13.1,6.5Hz,8H),2.52(m,2H),2.25(s,3H),1.91–1.83(m,2H),1.38(s,9H).
[0129] Step 3-2: Dissolve compound 3-B in 2 mL of dichloromethane and add 0.5 mL of trifluoroacetic acid. After reacting for 1 hour at room temperature, spin the system to obtain a solid...
Embodiment 9
[0130] Example 9: N-(2-((2,7-difluoro-3-oxo-9-(o-tolyl)-3H-xanthin-6-yl)amino)ethyl)-4-(hydroxy Methyl)-3-nitrobenzamide (9)
[0131]
[0132] Step 9-1: Dissolve 1-bromo-2,4,5-trifluorobenzene (3.5mL, 30mmol) in anhydrous tetrahydrofuran (THF), add isopropylmagnesium chloride (i-PrMgCl) at -78°C 2,4,5-Trifluorobenzaldehyde (2.9mL, 20mmol ). The resulting mixture was stirred for a further 10 min, then at room temperature for 24 h. Add saturated NH 4 The reaction was quenched with aqueous Cl, and Et 2 O extraction, the organic phase was Na 2 SO 4 Drying and evaporation gave crude product 9-A (6.7 g, 77%) as a brown oil. 1H NMR (500MHz, DMSO) δ7.63–7.43(m, 4H), 6.49(d, J=4.6Hz, 1H), 6.06(d, J=4.5Hz, 1H).
[0133] Step 9-2: CH to 9-A (6.6g, 22.6mmol) 2 Cl 2 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) (117mg, 1.13mmol), KBr (540mg, 4.5mmol), NaHCO 3 (3.8 g, 45.2 mmol), saturated aqueous NaCl solution, the reaction mixture was vigorously stirred, then aqueous NaOCl so...
Embodiment 21
[0140] Example 21: 4-(Hydroxymethyl)-N-(5-((4-(morpholinomethyl)-2-oxo-2H-benzopyran-7-yl)oxy)pentyl )-3-nitrobenzamide (21)
[0141]
[0142] Step 21-1: 4-Bromomethyl-7-methoxycoumarin (1.0 g, 3.7 mmol) was dissolved in anhydrous DCM under N 2 Protection, dropwise addition of BBr at -78°C 3 (9.3mmol), continued stirring at -78°C for 0.5h, and warmed to room temperature overnight. After the reaction was complete, water was added to quench the reaction, filtered, and washed with DCM to obtain 925 mg of the crude product as a pale yellow solid, which was directly used in the next step.
[0143] Step 21-2: Dissolve compound 21-A (410mg, 2.35mmol) in 2-methoxyethanol, add morpholine (410mg, 4.7mmol), and reflux for 6h. After the reaction was complete, it was concentrated and column chromatographed to obtain compound 21-B (610 mg, 99%) as a yellow solid. 1H NMR (500MHz, DMSO) δ10.51(s, 1H), 7.76(d, J=8.7Hz, 1H), 6.79(dd, J=8.7, 2.3Hz, 1H), 6.71(d, J=2.3Hz, 1H),6.24(s,1H),3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com