New application of bacteroides fragilis and immune checkpoint inhibitor
A technology of Bacteroides fragilis, immune checkpoint, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the fermentation culture of Bacteroides fragilis
[0060] Streak inoculation of Bacteroides fragilis ZY-312 strain on blood plate, anaerobic culture for 48h. Observe the colony morphological characteristics, staining characteristics, size, club shape and distribution, etc.
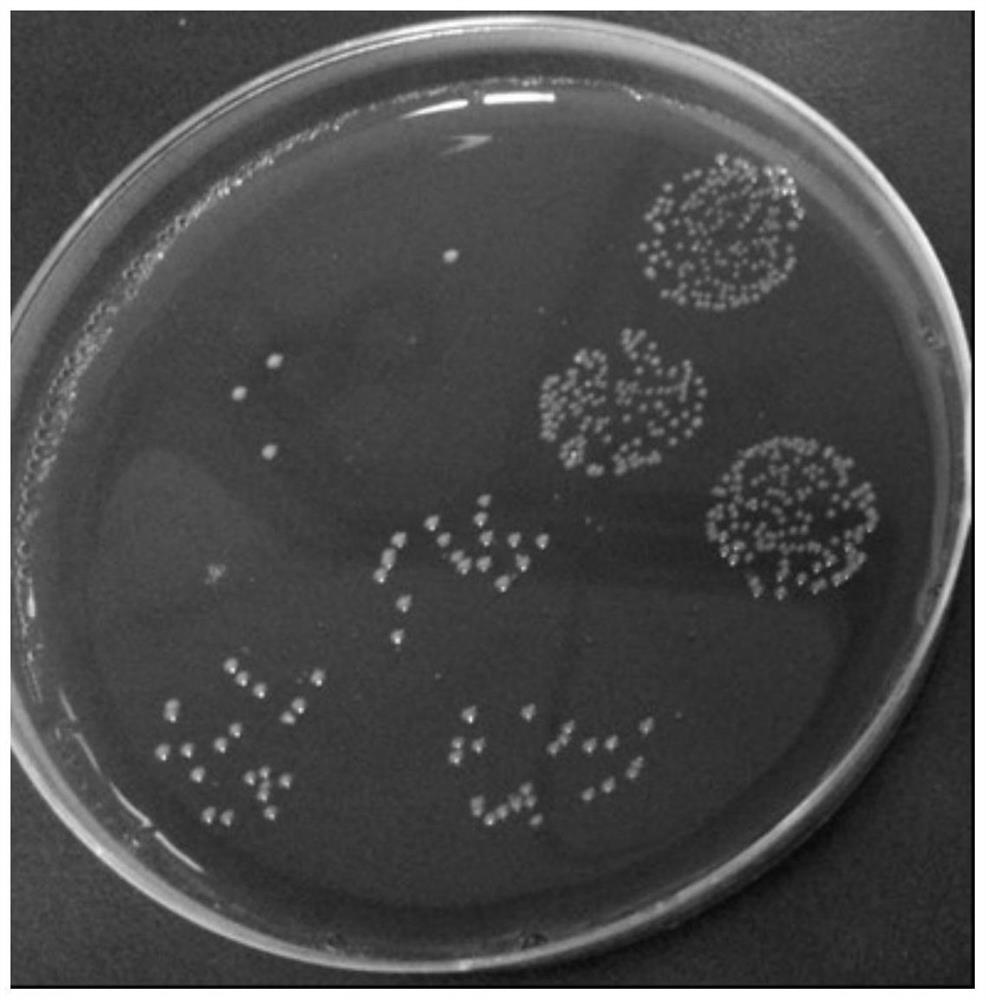
[0061] Colony characteristics: Bacteroides fragilis ZY-312, after being cultured on a blood plate for 48 hours, is slightly convex, translucent, white, smooth, non-hemolytic, and the diameter of the colony is between 1-3mm. See figure 1 .
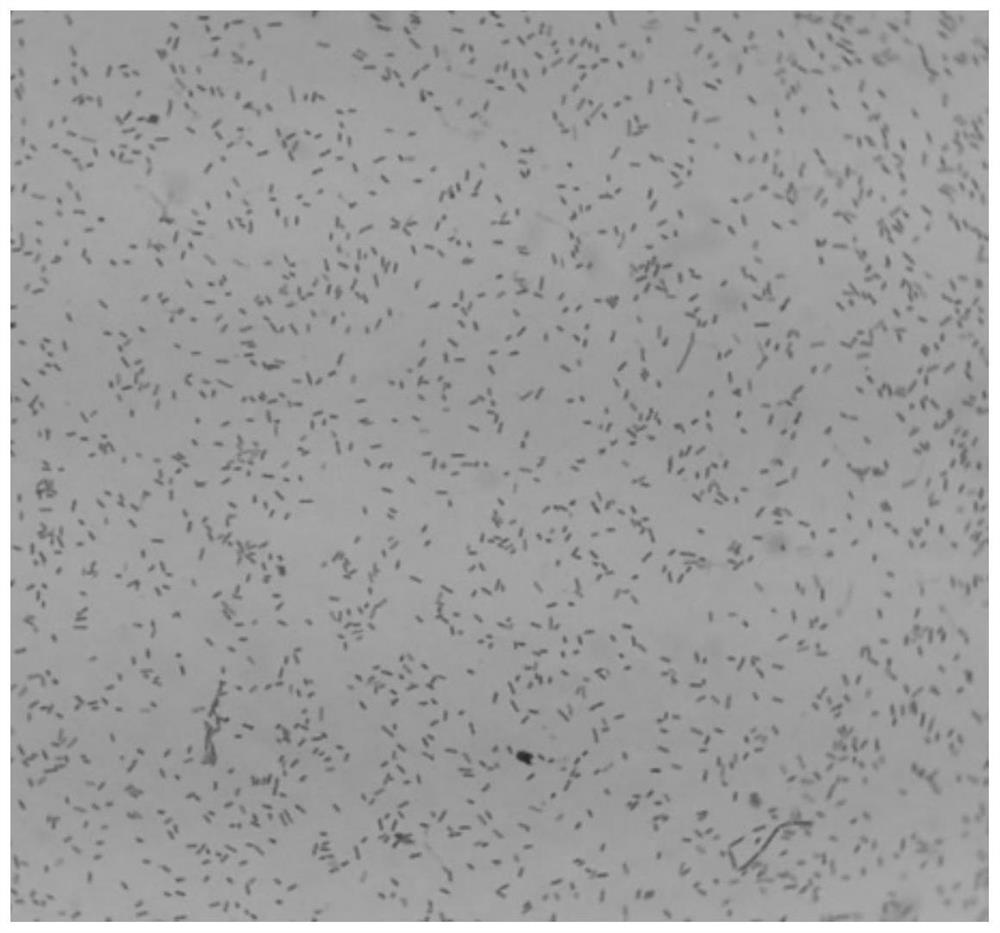
[0062] Morphology under the microscope: Bacteroides fragilis ZY-312 was examined by Gram staining. It is a Gram-negative bacterium with a typical rod shape, blunt rounded ends and dense staining. The uncolored part in the middle of the bacteria is like a vacuole. figure 2 .
[0063] Select a single colony and inoculate it in a plant-derived peptone liquid medium for fermentation and culture for 8 hours (at a temperature of 37°C). The obtained ba...
Embodiment 2
[0064] Embodiment 2: Preparation of Bacteroides fragilis live bacteria liquid
[0065] (1) Streak-inoculate the strain on a blood plate, and incubate anaerobically at 37°C for 48 hours.
[0066] Colony characteristics: Bacteroides fragilis ZY-312, after being cultured on a blood plate for 48 hours, is slightly convex, translucent, white, smooth, non-hemolytic, and the diameter of the colony is 1-3mm.
[0067] (2) Bacterial enrichment: select a single colony from step (1) and inoculate it in TSB (tryptone soybean broth, containing 5% fetal bovine serum) for enrichment culture, and the obtained bacterial solution is preserved for future use.
[0068] (3) Viable Bacteroides fragilis solution: the bacteria solution prepared in step (1) was measured with a McFarland turbidimetric tube and diluted to 10 with normal saline. 7 CFU / ml and 10 9 CFU / ml, save for future use.
Embodiment 3
[0069] Embodiment 3: Preparation of the inactivated bacteria powder of Bacteroides fragilis
[0070] (1) Get the Bacteroides fragilis fermented liquid that embodiment 1 makes and carry out centrifugation to fermented liquid, collect wet thallus, add physiological saline according to thalline: physiological saline=1:(10-30) (m:v) ratio The bacteria sludge was resuspended and washed, and the washed bacteria were collected by centrifugation again.
[0071] (2) Add the excipient that 5wt% maltodextrin+0.9wt% sodium chloride is mixed to step (1) gained thalline, press thallus: excipient=1:(5-15)(m:m ) ratio, stir and disperse, heat inactivate (20-40)±5 minutes at (70-100)±5°C to obtain inactivated bacteria solution.
[0072] (3) Centrifuge the inactivated bacteria liquid obtained in step (2) to collect the inactivated bacteria sludge.
[0073] (4) Add excipients to the inactivated bacteria slime collected in step (3) to make the total weight consistent with the weight of the bact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com