Method for measuring lectin-binding substance, kit for measuring lectin-binding substance, and block-labeled lectin used in said method and kit
An assay method and lectin technology, which is applied in the field of block-labeled lectin, can solve the problems of weak affinity reaction and difficulty in high-sensitivity assay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
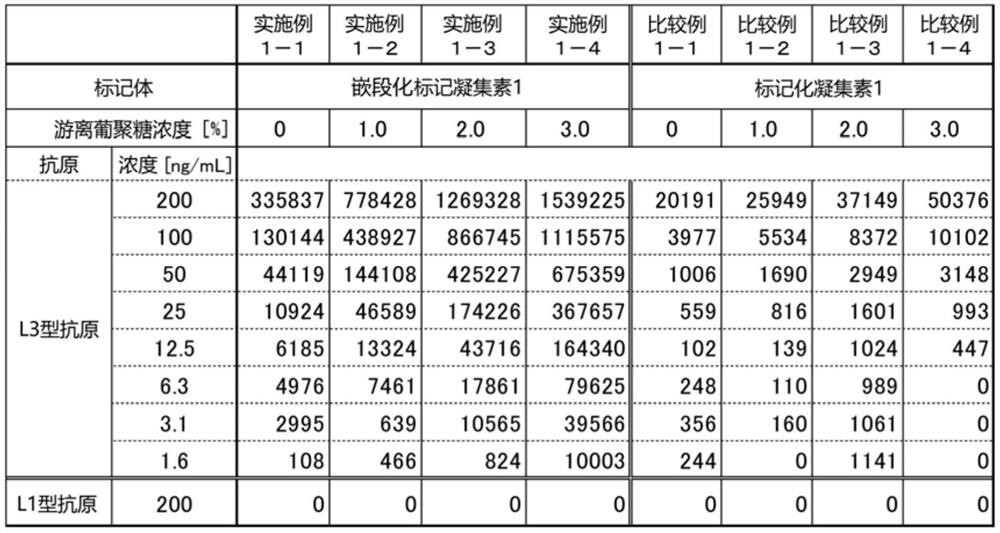
[0135] Determination of alpha-fetoprotein L3 fraction (AFP-L3) using block-labeled lectin and labeled lectin
[0136] (1) Preparation of hydrazine dextran
[0137] 240.0 mg of dextran with a molecular weight of 250K (manufactured by CarboMer) was added to 4.8 mL of 0.1 M phosphate buffer (pH 7.0), and stirred and dissolved in the dark at 25° C. for 30 minutes. Next, add 150mM NaIO 4 2.664 mL and 0.536 mL of ion-exchanged water were stirred in the dark at 25°C for 30 minutes. Using a PD-10 column (manufactured by GE Healthcare, Sephadex G-25 packed column), buffer exchange was performed with 0.1 M sodium phosphate buffer (pH 6.0) to obtain 20.0 mL of a solution. Add 5.04g of NH 2 NH 2 • HCl, stirred in the dark at 25°C for 2 hours. 800 mg of DMAB (dimethylamine borane) was added, and it stirred in the dark at 25 degreeC for 2 hours more. After performing dialysis with 4 L of ion-exchanged water in the dark using an RC50K (regenerated cellulose with a molecular weight of ...
Embodiment 2
[0159] Measurement of PSA derived from prostate cancer cells using block-labeled lectin and labeled lectin 1
[0160] (1) Use AAL to prepare block-labeled lectins
[0161] Except for using Dictyosporum amaranthus agglutinin (AAL; manufactured by VECTOR Co., Ltd.) instead of lentil agglutinin (LCA), it was carried out in the same manner as in Example 1 (1) to (5) to obtain 87.8 μg / mL 2.0 mL of fragmented labeled lectin 2 (dextran-enzyme-AAL conjugate) solution.
[0162] (2) Prepare labeled lectin using AAL
[0163] Except that the lentil agglutinin (LCA) was replaced with Dictyospermia aurantii agglutinin (AAL; manufactured by VECTOR Co., Ltd.), 34.4 μg / mL of labeled lectin 2 was obtained in the same manner as in (6) of Example 1. (ALP-AAL conjugate) solution 2.0 mL.
[0164] (3) Measurement of PSA derived from cancer cells
[0165] Magnetic particles (manufactured by Fuji Xerbio Co., Ltd.) and anti-PSA monoclonal antibody F(ab') 2 Fragments (manufactured by Fuji Xerbio Co...
Embodiment 3
[0172] Assay of AFP-L3 Using Multiple Blocked-Tagged Lectin Mixtures
[0173] (1) Preparation of block-labeled lectin
[0174] Except using dextran with a molecular weight of 500K (manufactured by Fluka Corporation) or dextran with a molecular weight of 70K (manufactured by TCI Corporation) as dextran, the same procedure as in (1) to (5) of Example 1 was performed to prepare each Block-tagged lectins. The block-labeled lectin using dextran with a molecular weight of 500K is referred to as "block-labeled lectin 3 (500K)", and the block-labeled lectin using dextran with a molecular weight of 70K is referred to as "block UL-tagged lectin 4 (70K)".
[0175] (2) Determination of AFP-L3 1
[0176]The composition of the particle dilution solution was set to 50mM Tris, 150mM NaCl, 2mM EDTA·2Na, 0.5mg / mL dextran sodium sulfate 5000, 30μg / mL mouse KLG, 1.0% BSA, 0.005% Antiform SI, 0.1% ProClin 300 , pH 7.2, except that, proceed in the same manner as in (7) of Example 1 to prepare a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com