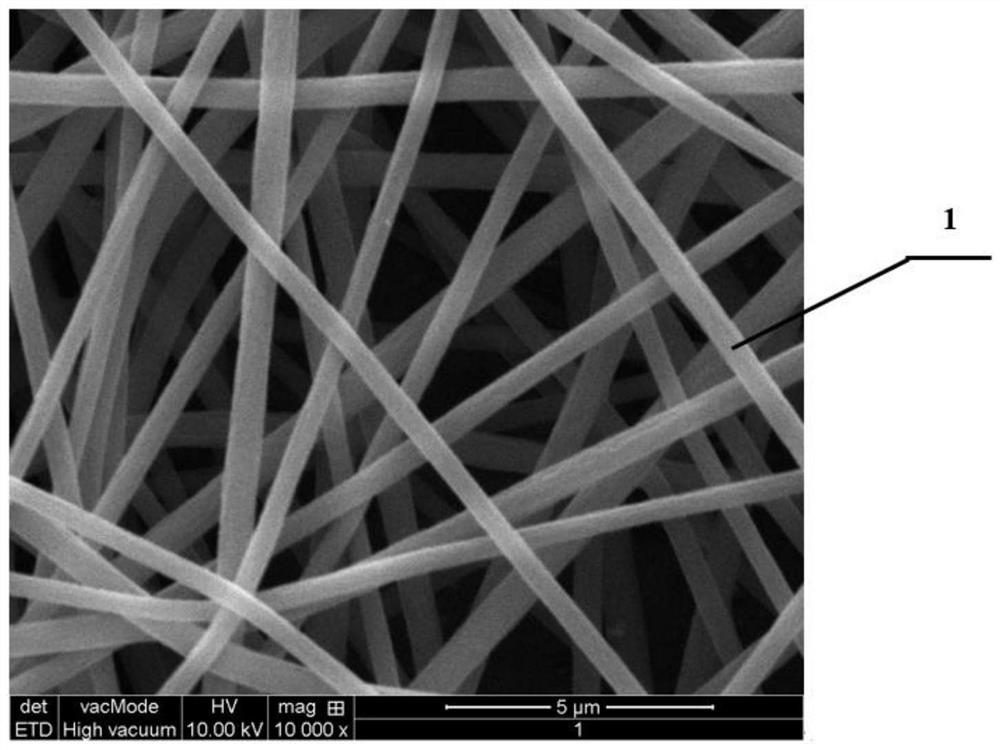
Drug-loaded absorbable anti-adhesion barrier and preparation method thereof
An anti-adhesion and barrier technology, which is applied in the fields of medical science, surgery, cellulose/protein conjugated artificial filament, etc., can solve the problems of non-environmental protection, high cost of preparation process, unstable degradation performance of anti-adhesion products, etc., and achieve reduction Degradation burden, effect of reducing thickness and dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Bacterial cellulose is prepared by the fermentation of acetic acid bacteria. Its appearance is a round or oval thin wet film with a diameter of 1 meter and a thickness of 2 mm.
[0051] The regeneration process of bacterial cellulose is pre-dissolved with sodium hydroxide / urea solvent system at a volume ratio of 1:20 at low temperature, and then the solution is slowly coagulated in a coagulation bath formed by 5g / L sulfuric acid solution through microfluidic technology for 5min , to form regenerated cellulose, which is then fully washed with water, freeze-dried, and ground into regenerated cellulose powder.
[0052] The oxidation process of regenerated bacterial cellulose is to carry out TEMPO carboxylation (TEMPO reagent 5g / L, temperature 50 ℃, time 6h) and sodium periodate (NaIO 4) formylation (reagent 5g / L, temperature 50°C, time 6h) modification to improve its surface activity, and then use carbon tetrachloride / nitrogen dioxide mixed solvent system (dinitrogen tetro...
Embodiment 2
[0062] Bacterial cellulose is produced by the fermentation of acetic acid bacteria. Its shape is a round or oval thin wet film. The diameter of the wet film is about 0.5 meters, and the thickness of the wet film is 0.5 mm.
[0063] The regeneration process of bacterial cellulose is pre-dissolved with sodium hydroxide / urea solvent system at a volume ratio of 1:20 at low temperature, and then the solution is slowly coagulated in a coagulation bath formed by 6g / L sulfuric acid solution through microfluidic technology for 8 minutes , to form regenerated cellulose, which is then fully washed with water, freeze-dried, and ground into regenerated cellulose powder.
[0064] The oxidation process of regenerated bacterial cellulose is to carry out TEMPO carboxylation (TEMPO reagent 5g / L, temperature 50 ℃, time 6h) and sodium periodate (NaIO 4 ) Formylation (reagent 5g / L, temperature 50°C, time 6h) modification to improve its surface activity, and then further fully oxidized (temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com