Polypeptide with PINK1 kinase agonist activity and application thereof
An active, dl-pt-1 technology, applied in the field of biochemistry, can solve the problems of clinical application limitations and achieve non-cytotoxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Separation and purification of polypeptide components
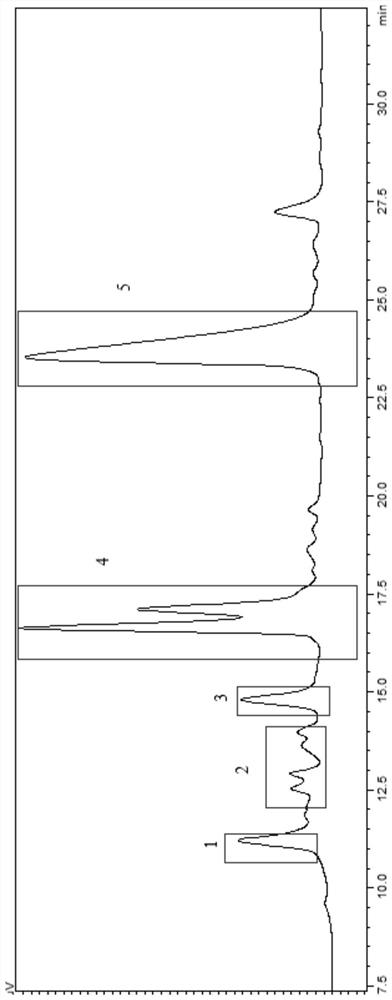
[0026] 1. Guided by the determination of PINK1 enzyme activity, five polypeptide components with anti-Parkinson potential activity were isolated and purified from the extract of Earthworm. The specific separation method is as follows: the dried earthworm is crushed and soaked in 80% methanol for extraction, and the extract is concentrated under reduced pressure to obtain the earthworm extract. Sephadex LH-20 gel column was used to separate the earthworm extract by column chromatography to obtain four fractions: DL-A, DL-B, DL-C and DL-D; the DL-B fraction has PINK1 agonistic activity, Therefore, it is further separated by C-18 reverse phase column chromatography to obtain DL-B-1, DL-B-2, DL-B-3, DL-B-4, DL-B-5, DL-B-6 , DL-B-7, DL-B-8, DL-B-9 and DL-B-10 are ten parts in total; the DL-B-8 part has PINK1 agonistic activity, so preparative HPLC (C- 18 column) to separate and purify it, and finally obtain...
Embodiment 2
[0037] Example 2: Structural Identification of Polypeptide Components
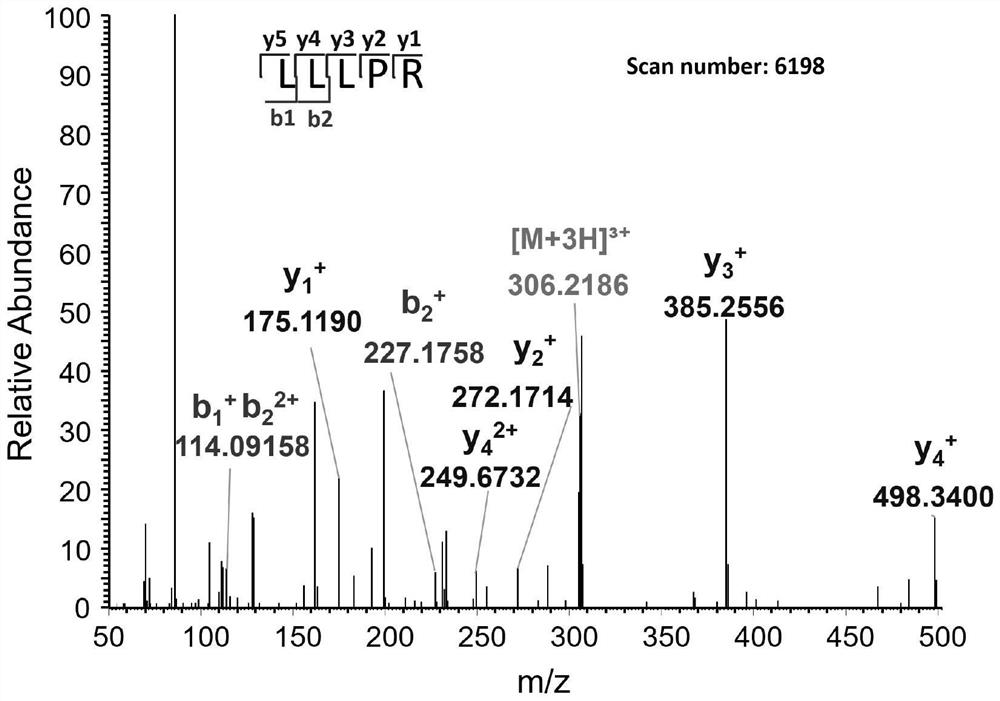
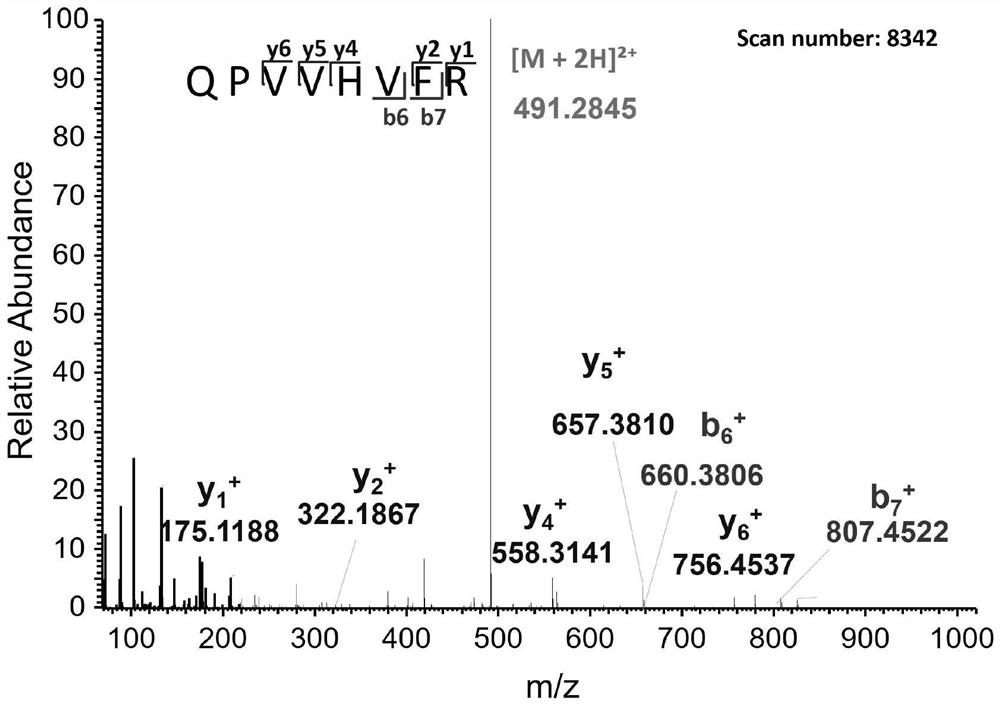
[0038] 1. Analyze the amino acid sequence of DL-B-8-c obtained in Example 1 by using a high-resolution liquid-mass spectrometry system (Q-Exactive-MS / MS), and analyze the mass spectrometry data with Proteomo Discoverver 1.4 and Peaks Pnovo , and using the polypeptide and protein sequence library of Dilong as the reference file for searching, 6 polypeptides were identified from DL-B-8-c, and the sequences are shown in Table 2. According to these six amino acid sequences, corresponding polypeptides were synthesized for subsequent testing of PINK1 agonistic activity and anti-PD activity.
[0039] Table 2 Comparison table of sequencing results of each polypeptide
[0040] polypeptide amino acid sequence Molecular weight (Da) DL-Pt-1 SEQ ID NO.1 610.4166 DL-Pt-2 SEQ ID NO.2 639.4319 DL-Pt-3 SEQ ID NO.3 1528.7271 DL-Pt-4 SEQ ID NO.4 1501.7512 DL-Pt-5 SEQ ID N...
Embodiment 3
[0045] Example 3: Cytotoxic activity test of polypeptide DL-Pt-6
[0046] Dilute the SH-SY5Y cell suspension at 1×10 4 The density of each well was inoculated in a 96-well plate, and 100 μL of complete medium (DMEM high-glucose medium containing 1% streptomycin / penicillin double antibody, 10% fetal bovine serum FBS) was added to each well, and the cell culture incubator ( 37°C, 5% CO 2 ) for 24 hours. After the cells enter the logarithmic growth phase (adhesive growth to 70%-80%), replace the medium with 100 μL of complete culture containing different concentrations of drugs (0.01, 0.05, 0.1, 0.5 μM DL-Pt-6) base, and continue to culture for 24 h. After the cultivation, the CCK8 kit was used to detect the absorbance at 450 nm, and the cell survival rate was calculated.
[0047] The formula for calculating the cell survival rate is: cell survival rate (%)=[(A-B) / (C-D)]×100%. Wherein A is the absorbance of the drug group (containing cells and the complete medium containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com