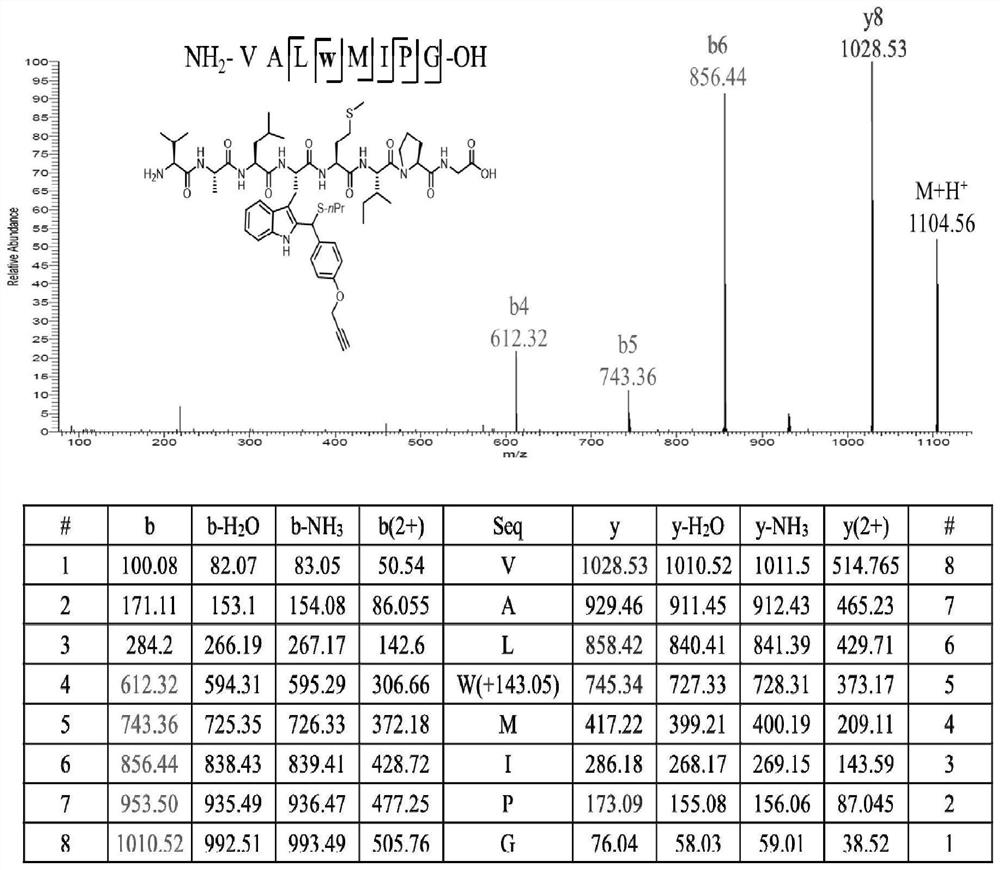
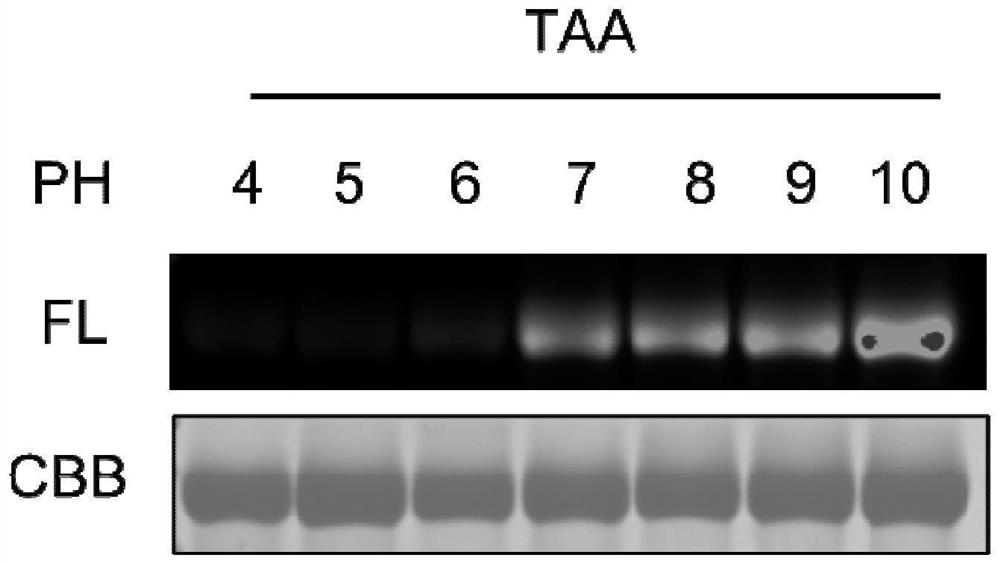
Method for modifying protein tryptophan residues
A protein and tryptophan technology, applied in the field of biochemistry, can solve problems such as difficult modification of aromatic amino acids, and achieve remarkable technological progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
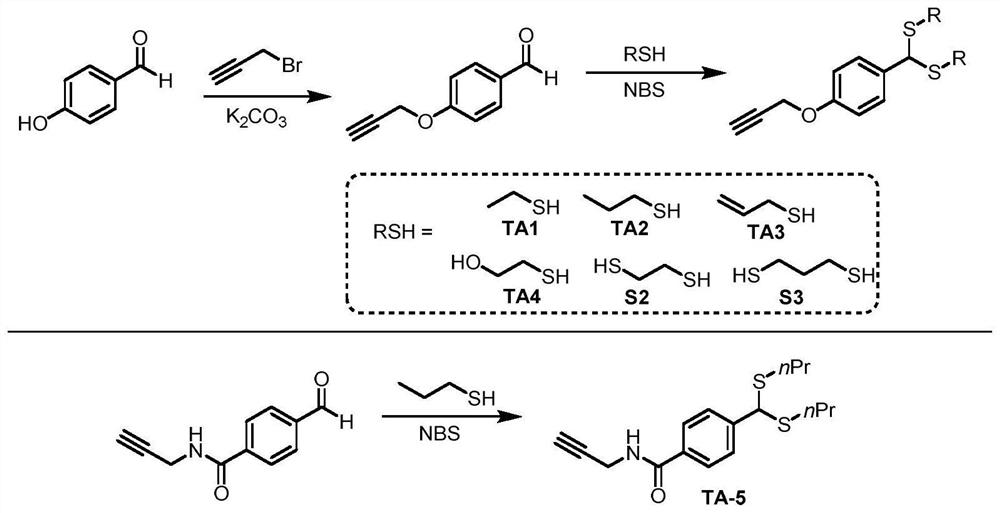
[0031] 1. Synthesis of Thiol Substrates
[0032] 12.2 g of 4-hydroxybenzaldehyde and 11.8 g of propyne bromide were added to a round bottom flask and dissolved with 300 mL of ethanol. 13.8 g of anhydrous potassium carbonate was added to the reaction system, and heated under reflux in an oil bath for 6 hours. After the reaction, the organic solvent was removed by rotary evaporation, and 500 mL of water was added to dilute the obtained viscous mixture. The aqueous solution was extracted with 100 mL×3 ethyl acetate, the organic phases were combined, washed with 100 mL×2 0.1M dilute hydrochloric acid and 100 mL×2 saturated brine, dried over anhydrous sodium sulfate, and the organic solvent was removed by rotary evaporation. The crude product was recrystallized from petroleum ether and ethyl acetate, and the resulting white solid was filtered and dried, which was the product 4-propargyloxybenzaldehyde (13.9 g, yield 87%).
[0033] 1 H NMR (300MHz, Chloroform-d) δ9.84(s, 1H), 7.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com