Medicine for treating infantile asthma
A technology for pediatric asthma and medicine, applied in the field of medicine, can solve the problem of difficult to apply medicine to infants and young children, and achieve the effect of avoiding oral or spray medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: preparation pharmaceutical preparation
[0022] Take 100g of silica gel, 400mg of albuterol, 3g of oleic acid, and 10mg of rose essential oil. First, mix the oleic acid and rose essential oil evenly, then add albuterol, mix well, and roll the silica gel with an open mill to form a special powder. Silica gel.
[0023] Cut the special silica gel into strip-shaped silica gel sheets, the length of the strip-shaped silicone rubber sheets is 240mm, the width is 5mm-50mm, the thickness is 1mm-2mm, and each strip of silica gel sheet weighs 5.5g.
Embodiment 2
[0028] Embodiment 2: drug stability test
[0029] The pharmaceutical preparation obtained in Example 1 was placed in an environment of 25°C and RH60% according to the commercially available packaging, and samples were taken at the 0, 3, 6, 9, 12, 18, and 24 months respectively to check for salbutamol, related substances, etc. Quality indicators, the results are shown in Table 1.
[0030] Table 1 Long-term test results of pharmaceutical preparations
[0031] time (month) Salbutamol content (%) relative substance(%) 0 100 0.18 3 100.89 0.24 6 100.12 0.28 9 100.04 0.30 12 100.31 0.34 18 100.49 0.35 24 100.84 0.42
[0032] The results show that the pharmaceutical preparation of the present invention is placed in an environment of 25° C. and RH60% for 36 months according to the commercially available package, and all indicators have no obvious changes compared with 0 months, and the quality is stable and reliable, which m...
Embodiment 3
[0033] Embodiment 3: pharmacokinetic experiment
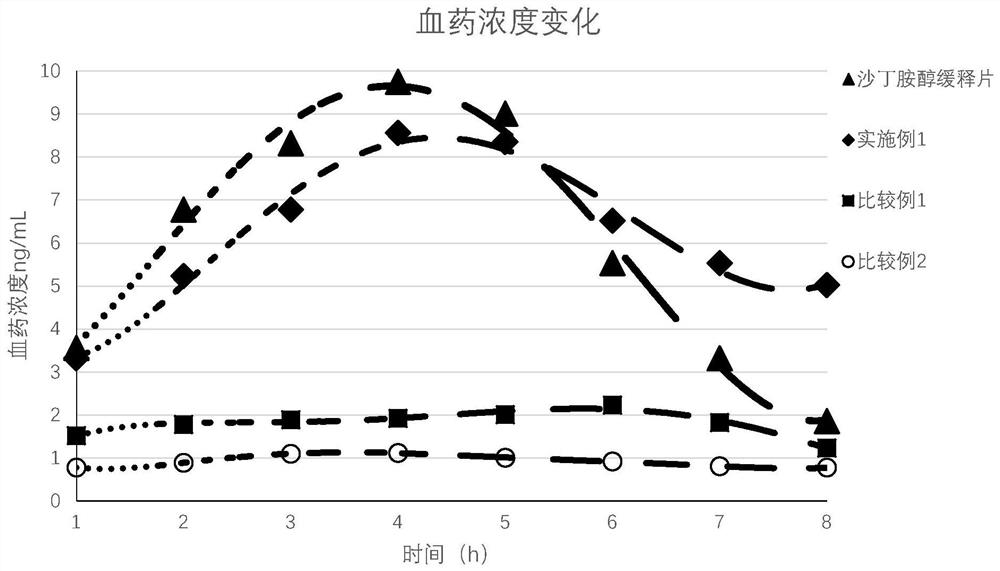
[0034] 40 healthy volunteers were divided into 4 groups, among which the silicone sheets of Example 1, Comparative Example 1 and Comparative Example 2 were worn on the left wrists as bracelets in three groups. Another group took commercially available albuterol sustained-release tablets (each containing 8 mg albuterol). Then, collect 1ml of venous blood every hour, and measure its salbutamol blood drug concentration, and the experimental results are as follows: figure 1 shown.
[0035] From the experimental results, the four groups of T max All between 4 hours and 5 hours, but the blood concentration of Comparative Example 1 and Comparative Example 2 is obviously low, which shows that it is difficult for albuterol to achieve Adequate transdermal absorption. Compared with the oral albuterol sustained-release tablets, the blood concentration of Example 1 is slightly lower within 1-5 hours, but the blood concentration is high...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap