Mutant of 5-aminolevulinic acid synthase from Rhodobacter capsulata and its application
A technology of aminolevulinic acid and capsulated rhodobacter, applied in the direction of bacteria, application, acyltransferase, etc., can solve the problems affecting the synthesis of 5-ALA, and achieve the effect of high relative enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1. Construction of Rhodobacter capsularis 5-ALA synthetase mutant
[0037] Select Rhodobacter capsulata in the KEGG database ( R. capsulatusla SB1003) sourced from hemA The gene is used as a template, and the codon is optimized according to the codon preference of the host cell Corynebacterium glutamicum, and the nucleotide sequence shown in SEQ ID NO.9 is synthesized in Jinweizhi Company. Using this sequence as a template, using SEQ ID NO.3 / SEQ ID NO.5, SEQ ID NO.6 / SEQ ID NO.4 as upstream and downstream primers to amplify fragment 1 and fragment 2 respectively, and then using SEQ ID NO. 3 / SEQ ID NO.4 is the primer for fusion PCR amplification of fragment 1 and fragment 2 to obtain the gene hemA The PCR fragment whose cysteine (C) at position 201 is mutated into alanine (A) is denoted as fragment 3, and its nucleotide sequence is as shown in SEQ ID NO.2 (the gene encoding is as shown in SEQ ID NO.1 5-ALA synthetase mutants shown). Using SEQ ID NO.3 / ...
Embodiment 2
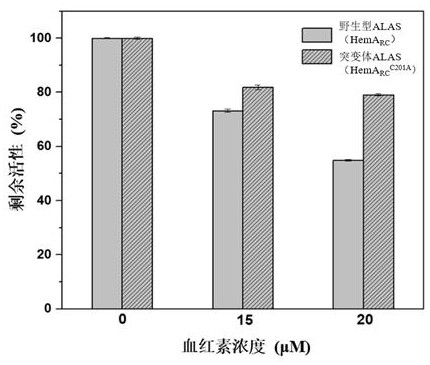
[0043] Example 2. Detection of Enzymatic Properties of Rhodobacter capsularis 5-ALA Synthetase Mutant
[0044] The wild-type plasmid pET28a-RC and the mutant plasmid pET28a-RC-C201A were transformed into E. coli BL21, the strains BL21-pET28a-RC and BL21-pET28a-RC-C201A were obtained.
[0045] (1) Bacteria culture: Inoculate the above-mentioned single recombinant colonies into 5 mL of LB liquid medium containing 40 μg / mL kanamycin, and culture at 37°C and 220 rpm for 12 hours. Transfer to 5 mL of LB liquid medium containing 40 μg / mL kanamycin. After the seeds grow thick, transfer to a 1L Erlenmeyer flask containing 200 mL of LB liquid medium containing 40 μg / mL kanamycin. Waiting for OD 600 At 0.6-0.8, add IPTG with a final concentration of 0.5mM, induce the temperature at 16°C, and induce the time for about 14 hours, centrifuge at 4500rpm, remove the supernatant, and collect the bacteria.
[0046] (2) Protein purification:
[0047] Binding buffer A: 25mM Tris, 150mM NaC...
Embodiment 3
[0061] Embodiment 3. Capsulated Rhodobacter 5-ALA synthetase mutant is on the influence of 5-ALA synthesis in Corynebacterium glutamicum
[0062] Using plasmids pET28a-RC and pET28a-RC-C201A as templates, and using SEQ ID NO.7 / SEQ ID NO.8 as upstream and downstream primers, PCR amplification was performed to obtain fragment 5 and fragment 6, respectively. Fragment 5, fragment 6 and plasmid vector pXMJ19 (commercial product) were double digested with restriction endonuclease HindIII / XbaI respectively, and the digested fragments and plasmid were respectively connected, and the plasmid pX-RC was finally obtained after the sequencing was correct. , pX-RC-C201A. The constructed plasmids were respectively electroporated into the Corynebacterium glutamicum engineering strain CB8 constructed in the early stage of the laboratory (the source of Corynebacterium glutamicum CB8 is detailed in Chinese patent CN106636171 A, the invention name is: production of 5-aminolevulinic acid glutamic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com