Application of biomarker in preparation of gestational diabetes diagnostic reagent
A biomarker and gestational diabetes technology, applied in the field of medical diagnosis, can solve the problems of unclear pathogenesis of GDM, not conducive to improving maternal and child outcomes, and poor patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
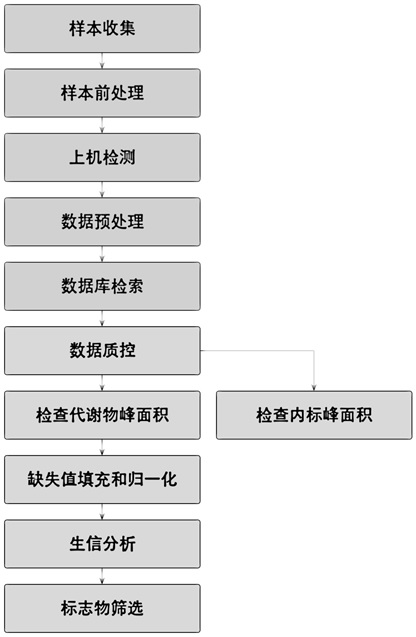
[0118] Example 1: Collection of Serum Samples
[0119]Serum samples from normal pregnant women and gestational diabetes were collected from 30 individuals in the second trimester (20-28 weeks) of pregnancy and confirmed by the gold standard test.
Embodiment 2
[0120] Example 2: Extraction of serum metabolites
[0121] According to the ratio of 1:4, methanol precipitant containing various isotope internal standards was added to the serum sample, shaken for 3 minutes, and then centrifuged at 4000 × g for 10 minutes at 20 °C. Take 4 parts of 100 μL supernatant from each sample to 4 sample plates, blow dry with nitrogen, and add various complex solutions containing isotopic internal standards for subsequent UPLC-MS / MS detection.
Embodiment 3
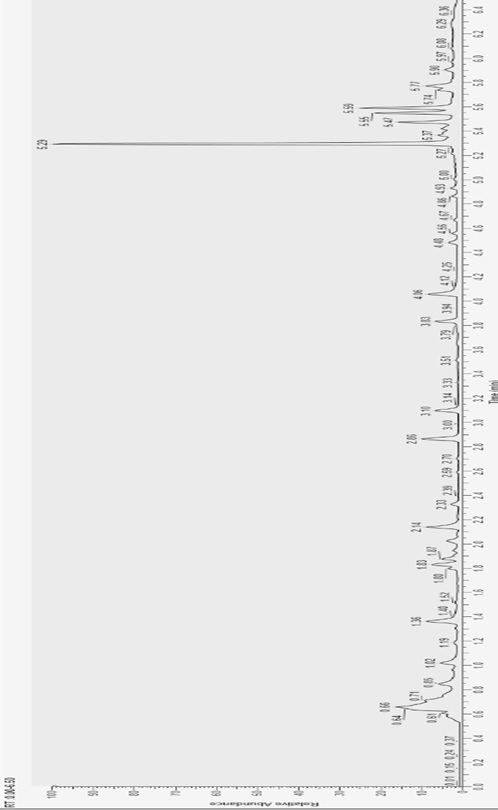
[0122] Example 3: Detection of extracted serum metabolites and data preprocessing
[0123] (1) Liquid chromatography / mass spectrometry conditions
[0124] The four UPLC-MS / MS methods were performed using ACQUITY 2D UPLC (Ultra Performance Liquid Chromatography; Waters, Milford, MA, USA) coupled with Q Exactive (QE) high-resolution mass spectrometry (Thermo Fisher Scientific, SanJose, USA). The mass spectrometer parameters are: scanning resolution 35000, scanning range 70-1000 m / z.
[0125] The specific parameters of the four UPLC-MS / MS methods are as follows:
[0126] Method 1: QE was detected by positive ion electrospray ionization (ESI) mode, and the liquid phase was separated by a C18 chromatographic column (UPLCBEH C18, 2.1x100 mm, 1.7 μm; Waters), and the mobile phase was 0.05% PFPA (pentafluoropropane anhydride) and 0.1% FA (formic acid) in water (A) and methanol (B);
[0127] Method 2: QE was detected by negative ion electrospray ionization (ESI) mode, and the liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com