Preparation method of high-purity nitrendipine bulk drug
A technology of high-purity nitrendipine and nitrendipine, which is applied in the field of preparation of high-purity nitrendipine raw materials, can solve the problems of impurity B and impurity C control, and the quality data of nitrendipine is not given, so as to avoid impurities B and Formation of Impurity C, High Yield, Effect of Guaranteed Yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
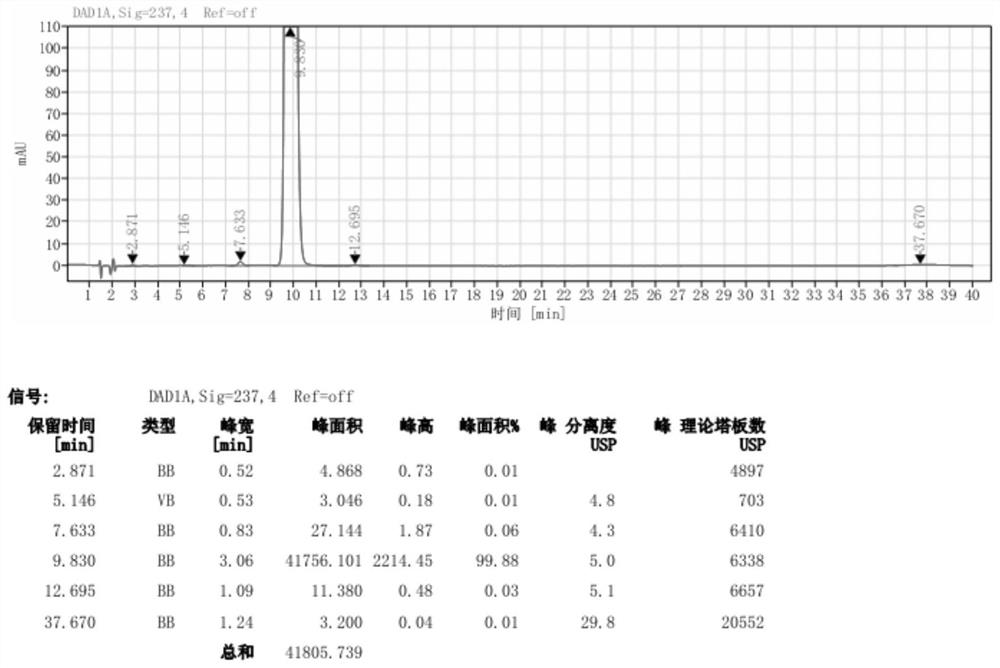
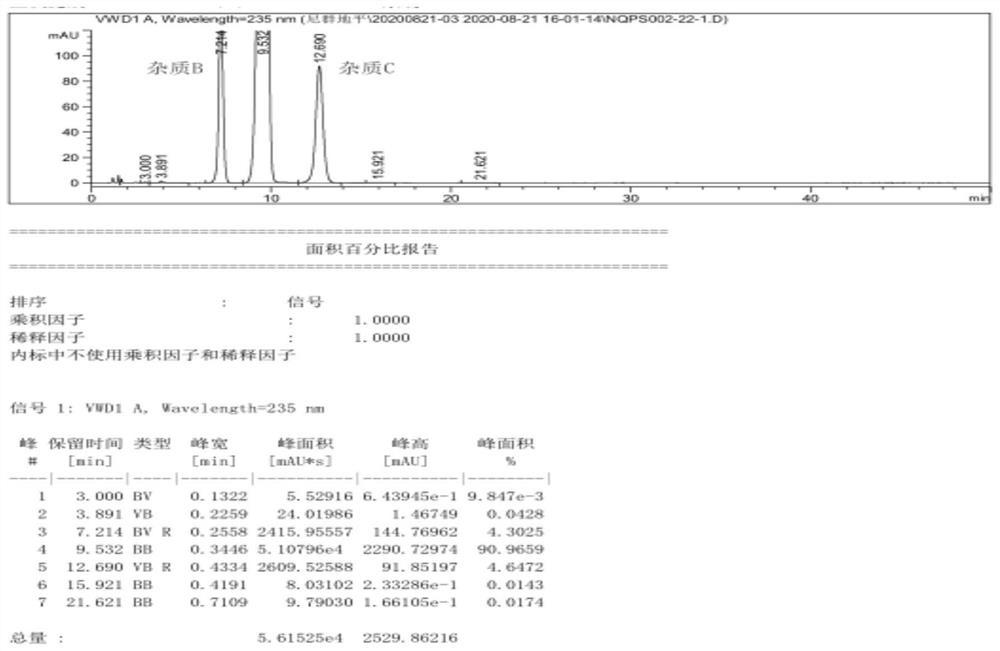
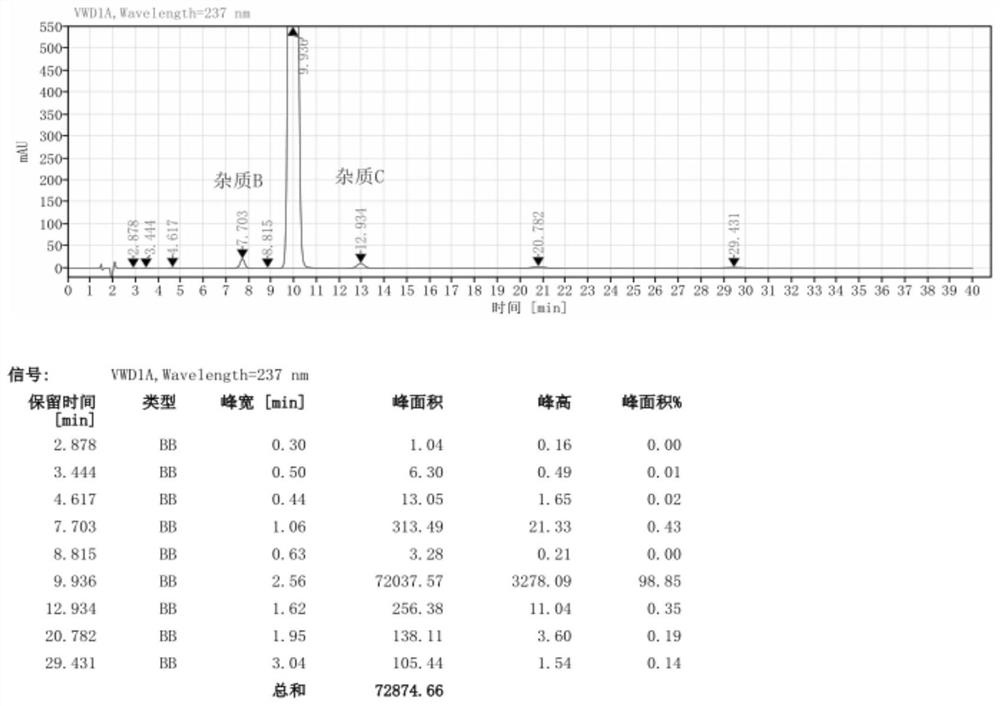
[0057] Pump 90.0kg of ethyl acetoacetate and 5.0kg of 98% concentrated sulfuric acid into a 1000L kettle, add 100.0kg of m-nitrobenzaldehyde in batches, heat up during the feeding process, and stir the reaction at 20-25°C after the addition is complete 2 to 8 hours; the reaction system is gradually solidified, and after the completion of the reaction, the crude product 2-(3-nitrobenzylidene)ethyl acetoacetate is obtained; add 200kg of water to the reaction kettle for beating and dispersion, and after stirring for 20 minutes, discharge the material for centrifugation Shake off the filter, centrifuge the solid and beat it with 200kg of water to disperse it, centrifuge again, and dry the centrifuged solid at 40-45°C for 12 hours to obtain about 155.0kg of pure ethyl 2-(3-nitrobenzylidene)acetoacetate (content 98.74%, yield 89.10%);
[0058] Add 150.0kg of ethyl 2-(3-nitrobenzylidene)acetoacetate to 480.0kg of ethanol, then add 85.0kg of methyl 3-aminocrotonate, and then add 30.0k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com