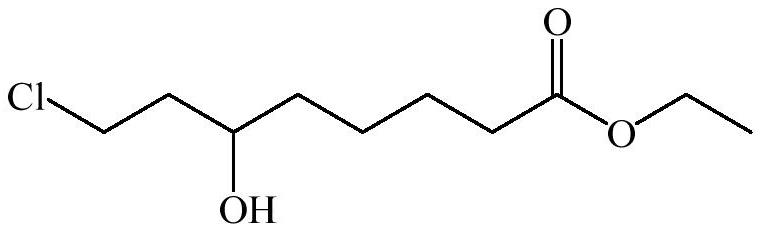
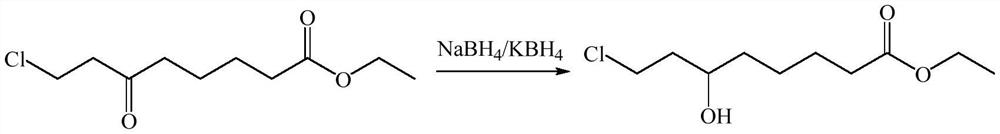
Synthetic method of 6-hydroxy-8-chloro ethyl caprylate, 6, 8-dichloro ethyl caprylate and lipoic acid
A technology of ethyl dichlorooctanoate and ethyl chlorooctanoate, applied in the field of organic synthesis, can solve problems such as low yield, and achieve the effects of low cost, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Under a nitrogen atmosphere at room temperature, a dichloroethane solution (150 g, 15% w / w) of 6-oxo-8-chlorooctanoic acid ethyl ester was poured into a reaction kettle with a rectifying tower, and lithium chloride (0.85 g, 0.2 equivalents), potassium phosphate (21.23 g, 1.0 equivalents) and isopropanol (38 mL, 5.0 equivalents), stirred for 10 min, heated to 83-85° C. and refluxed for 6 hours. During the reaction, the isopropanol-dichloroethane is separated from the acetone produced by the reaction through a rectification tower, and the isopropanol-dichloroethane flows back into the reactor to separate the acetone. The reaction was tracked by thin-layer chromatography. After the reaction was completed, the excess isopropanol and dichloroethane were distilled off under reduced pressure, and then the solids in the system were removed by filtration, and then the product 6-hydroxyl-8- Ethyl chlorooctanoate 19.92g, the content is 98.3%, the yield is 88.2%.
Embodiment 2-5
[0041] Synthesize 6-hydroxyl-8-chlorooctanoic acid ethyl ester according to the method of Example 1, the difference is that the amount of catalyst and the reaction time are changed according to the manner in Table 1, and the remaining conditions are the same as in Example 1 to obtain 6-hydroxyl-8-chlorooctanoic acid Ethyl esters, the results are shown in Table 1.
Embodiment 6
[0055] Embodiment 6 changes the consumption of Virahol and the reaction time
[0056] Synthesize 6-hydroxy-8-chlorooctanoic acid ethyl ester according to the method of Example 1, the difference is that the amount of isopropanol is adjusted from 5.0 equivalents to 4.0 equivalents, and the reaction time is adjusted to 10 hours, and the remaining conditions are the same as in Example 1 Same, the specific steps are as follows:
[0057] Under a nitrogen atmosphere at room temperature, a dichloroethane solution (150 g, 15% w / w) of 6-oxo-8-chlorooctanoic acid ethyl ester was poured into a reaction kettle with a rectifying tower, and lithium chloride (0.85 g, 0.2 equivalents), potassium phosphate (21.23 g, 1.0 equivalents) and isopropanol (24 mL, 4.0 equivalents), stirred for 10 min, heated to 83-85 ° C for 10 hours under reflux. During the reaction, the isopropanol-dichloroethane is separated from the acetone produced by the reaction through a rectification tower, and the isopropano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com