Method for synthesizing precursor of vilanterol intermediate
A time and compound technology, applied in the field of synthesizing a precursor for synthesizing a vilanterol intermediate, can solve the problems of long synthetic route steps and low atom economy, and achieve high asymmetric selectivity and high atom economy. , the effect of shortening the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069]
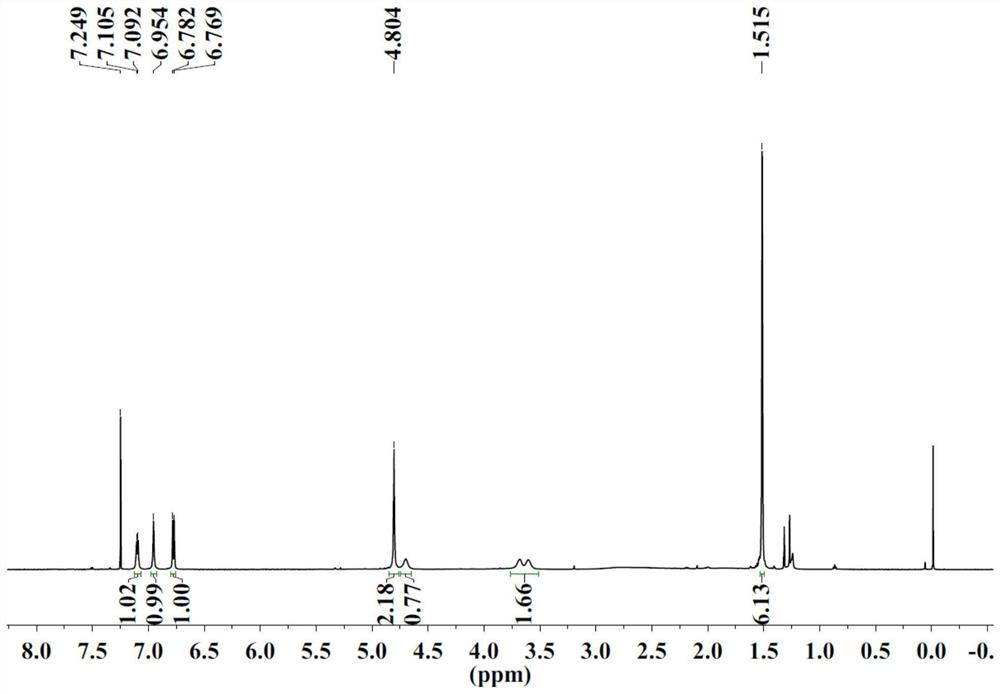
[0070] S1: SN2 substitution reaction to prepare α-nitroketone compound III
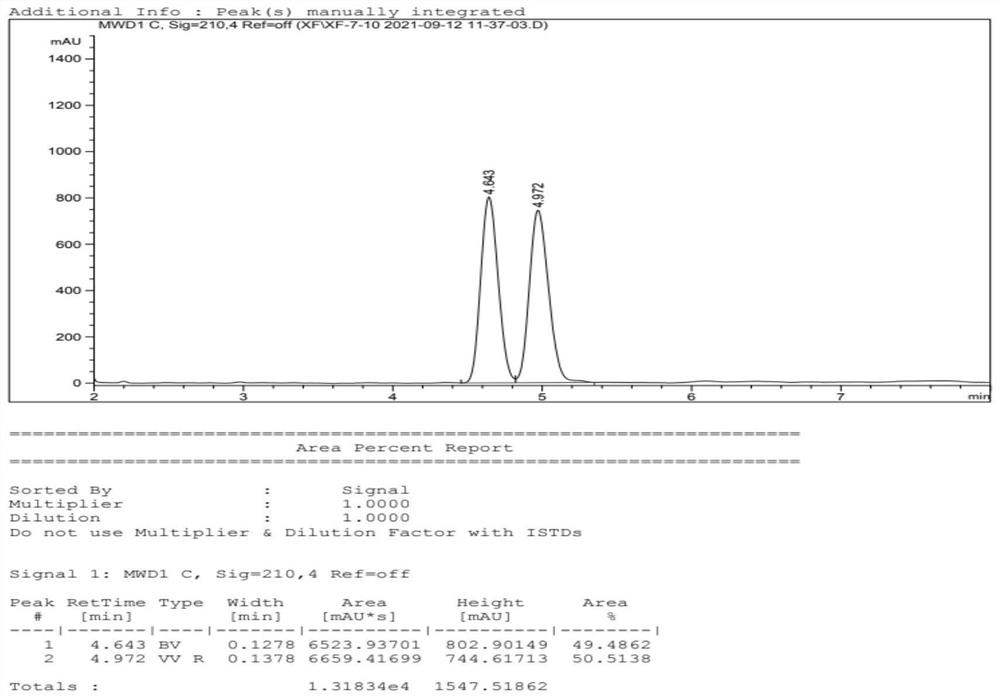
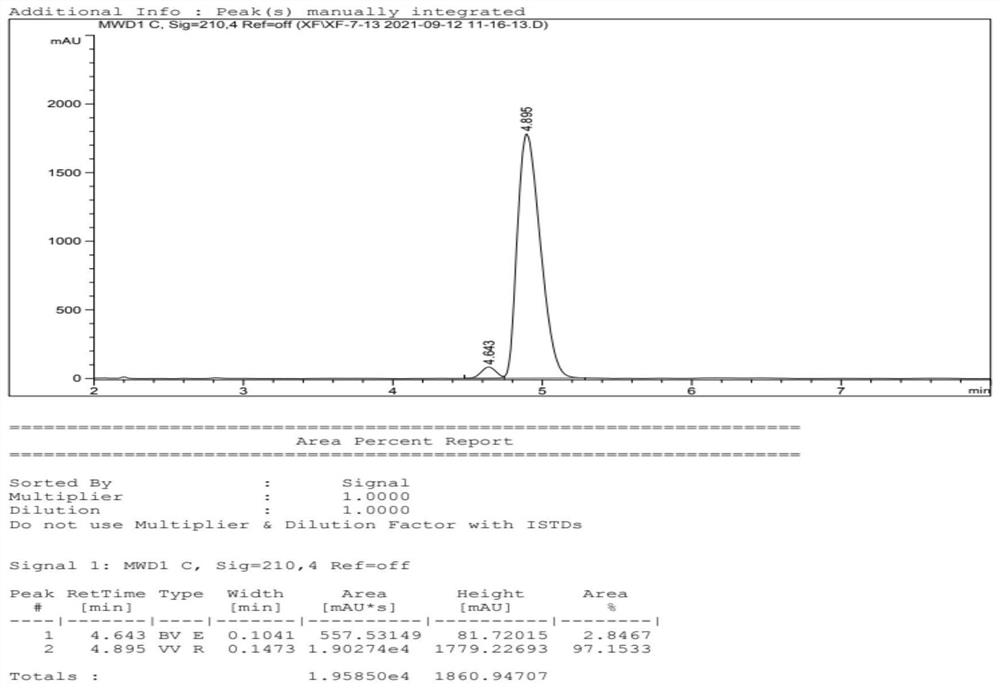
[0071] Add water, compound II (285g), imidazole-based polyionic liquid catalyst (0.1g, structural formula as follows), sodium nitrite (104g, 1.5eq.) into the reaction flask, heat up to 60°C and stir. After reacting for 20 min, stop stirring. Diethyl ether was added to extract three times, and the resulting organic phases were combined and dried over anhydrous sodium sulfate. After filtering, the organic phase was concentrated, and the concentrate was purified by silica gel column chromatography to obtain 176 g of compound III with a yield of 70%.
[0072]
[0073] Structural Formula 1: Structure of imidazole-based polyionic liquid catalyst
[0074] S2: Preparation of α-Nitroalcohol Compound I by Catalytic Transfer Hydrogenation
[0075] Add formula III compound (10g, 1.0eq.), catalyst RuCl[(S,S)-TsDPEN](p-cymene) (1.25g, 0.05eq.), DMF (50mL ). Take triethylamine (8.7mL) and formic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com