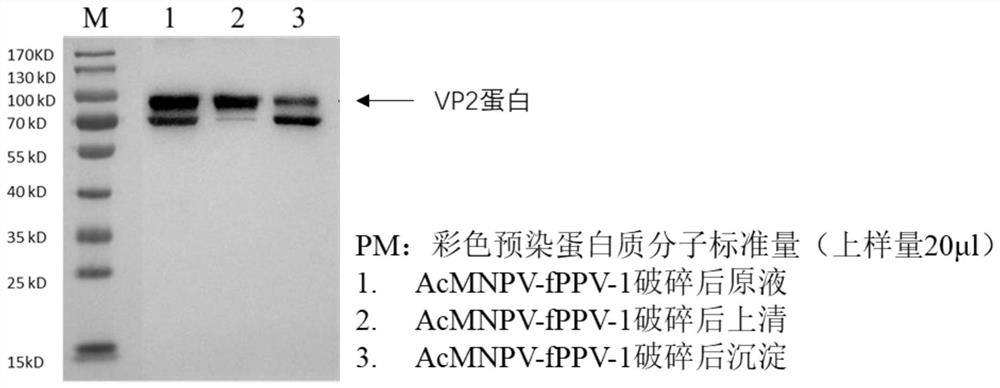
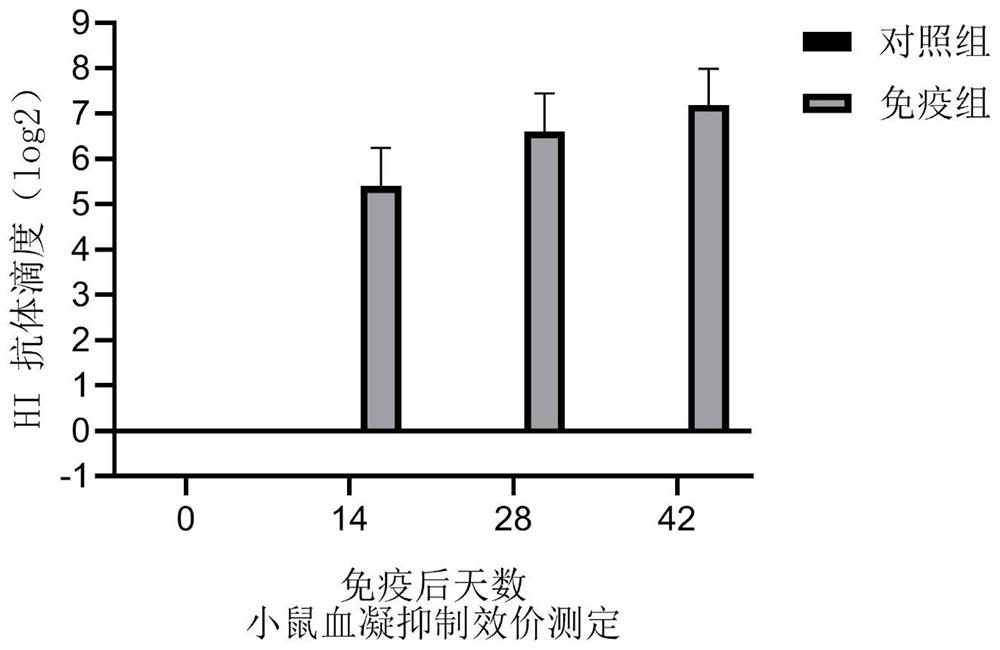
Recombinant baculovirus for expressing porcine parvovirus VP2 protein as well as preparation method and application of recombinant baculovirus
A technology for recombining baculovirus and parvovirus, applied in the biological field, can solve the problems of endotoxin contained in the product, unable to form correct spatial structure, affecting activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Construction and acquisition of recombinant vectors
[0034] Referring to the VP2 sequence of the PPV-NADL-2 strain gene (KF049424.1), the insect cell tropism was optimized, and the optimized sequence was added with a 6×His tag at the carboxy-terminal as SEQ ID No: 1, and the primers were designed using bioinformatics software. A secretory peptide and a kozak sequence were added to the N-terminus of the VP2 gene.
[0035] Upstream primer F1 (SEQ ID No: 3):
[0036] GTGTACATTTCTTACATCTATGCGGCCACCATGTCCGAGAATGT;
[0037] F2 (SEQ ID No: 4):
[0038] TAGTCAACGTTGCCCTTGTTTTTTATGGTCGTGTACATTTCTACATC;
[0039] F3 (SEQ ID No: 5):
[0040] CCG AGATCT ATGAAATTCTTAGTCAACGTTGCCCT (the underlined part is the BglⅡ restriction site);
[0041] Downstream primer R1 (SEQ ID No:6):
[0042] GAC GCGGCCGC CTAATGATGATGATGATGATGAT (the underlined part is the Not I restriction site).
[0043] The amplified VP2 sequence (SEQ ID No: 2) and the pVL1392 vector were digested with BglⅡ...
Embodiment 2
[0049] 1. Preparation of porcine parvovirus VP2 protein vaccine
[0050] The concentration of the VP2 protein purified in Example 1 was measured, filtered, and the ratio of antigen and Gel02 adjuvant was emulsified at a ratio of 4:1 to prepare a vaccine. The content of VP2 antigen per ml of vaccine was 20 μg.
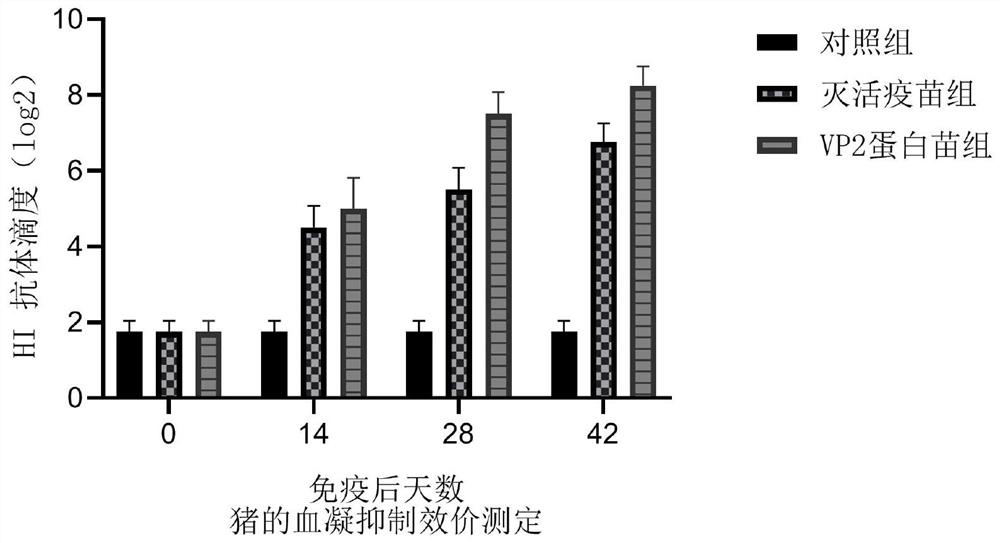
[0051] 2. Safety evaluation of porcine parvovirus VP2 protein vaccine
[0052] Ten female Balb / C mice weighing 16-18 g were divided into two groups, A and B, with 5 mice in each group. Each mouse in group A was subcutaneously injected with 0.3mL of the prepared VP2 protein vaccine; each mouse in group B was injected with 0.3ml of dialysis buffer (20mM Tris, 200mM NaCl); continuous observation for 14 days, the two groups of mice were in the same state and There was no abnormal reaction, which indicated that the vaccine was safe for mice.
[0053] Six PPV-negative pigs about 2 months old were randomly divided into two groups, A and B, with 3 pigs in each group. Each pi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com