Preparation method of tetrahydronaphthalene benzamide key intermediate
A technology of tetralin and intermediates, applied in the field of medicine, can solve the problems of long reaction time, complicated operation, low yield and the like, and achieve the effects of reducing by-products, shortening the reaction time, and improving time efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
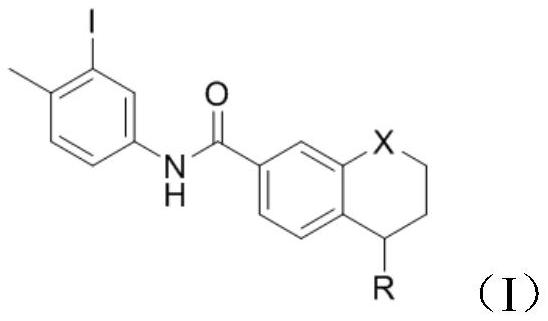
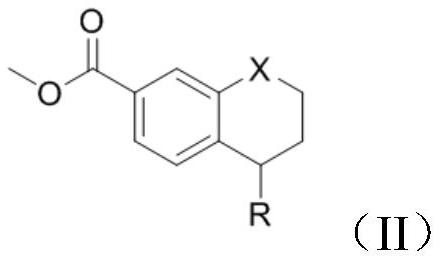
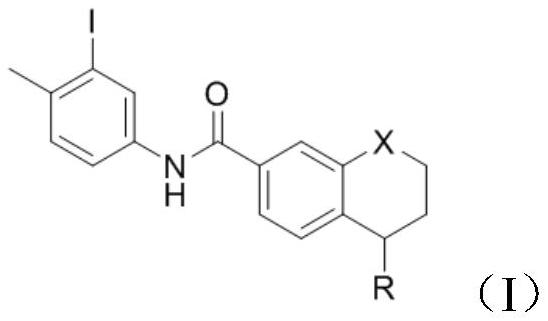
[0033] Add 5-(4-methylpiperazin-1-yl)-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid methyl ester (1g, 3mmol) into the reaction vessel, add 10mL of toluene to dissolve , control the temperature at 20°C, add 3-iodo-4-methylaniline (0.806g, 3.3mmol), and stir to dissolve. The resulting reaction system was evacuated, under nitrogen protection, and under stirring at room temperature, trimethylaluminum toluene solution (2.1ml, 2mol / L) was slowly added dropwise. After the addition was complete, the reaction system was heated to 100°C, reacted for 3h, and cooled to room temperature. Add 10 mL of water and 10 mL of dichloromethane to the resulting reaction mixture for extraction, separate the organic phase, wash with 5% aqueous potassium sodium tartrate solution twice, wash with water three times, wash with saturated brine once, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure and filter with suction to obtain 1.58 g of a white solid with a yield of 93.3% and...
Embodiment 2
[0037] Add 5-(4-methylpiperazin-1-yl)-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid methyl ester (1g, 3mmol) into the reaction vessel, add 10mL of toluene to dissolve , control the temperature at 20°C, add 3-iodo-4-methylaniline (0.806g, 3.3mmol), and stir to dissolve. The resulting reaction system was evacuated, under nitrogen protection, and under stirring at room temperature, trimethylaluminum toluene solution (2.1ml, 2mol / L) was slowly added dropwise. After the addition was complete, the reaction system was heated to 90°C, reacted for 3h, and cooled to room temperature. Add 10 mL of water and 10 mL of dichloromethane to the resulting reaction mixture for extraction, separate the organic phase, wash with 5% aqueous potassium sodium tartrate solution twice, wash with water three times, wash with saturated brine once, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure and filter with suction to obtain 1.52 g of a white solid with a yield of 90.2% and ...
Embodiment 3
[0039]Add 5-(4-methylpiperazin-1-yl)-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid methyl ester (1g, 3mmol) into the reaction vessel, add 10mL of toluene to dissolve , control the temperature at 20°C, add 3-iodo-4-methyl-aniline (0.806g, 3.3mmol), and stir to dissolve. The resulting reaction system was evacuated, under nitrogen protection, and under normal temperature stirring, trimethylaluminum toluene solution (2.1ml, 2mol / L) was slowly added dropwise. After the dropwise addition, the reaction system was heated to 80°C, reacted for 3h, and cooled to room temperature. 10 mL of water and 10 mL of dichloromethane were added to the resulting reaction mixture for extraction, the organic phase was separated and washed twice with 5% potassium sodium tartrate aqueous solution, three times with water, once with saturated brine, and dried over anhydrous sodium sulfate. Concentrate under reduced pressure and filter with suction to obtain 1.34 g of a white solid with a yield of 79.2% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com