Functional molecule with anti-tumor activity, preparation method and application thereof
A technology of anti-tumor activity and functional molecules, applied in the field of molecular synthesis, can solve the problems of normal tissue harm, threat to human health, lack of specificity and selectivity, etc., achieve high specificity and high selectivity to kill tumor cells, improve The level of reactive oxygen species in tumor cells and the effect of stereoselective type
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
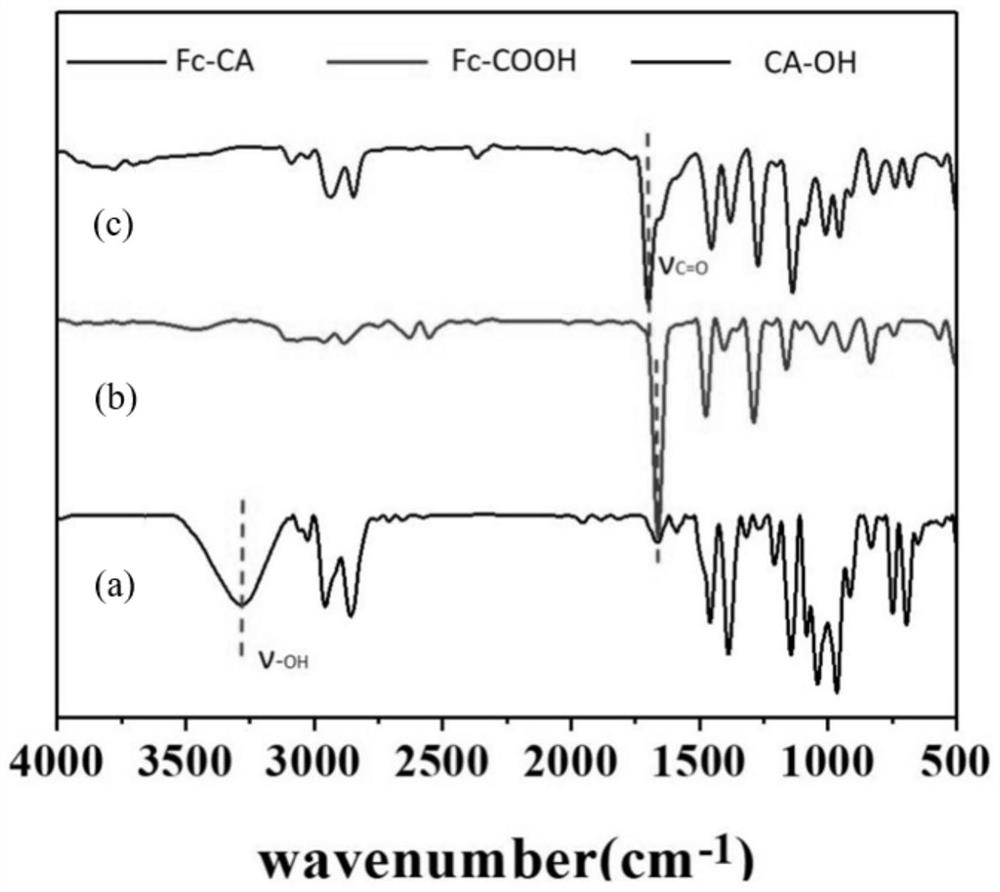
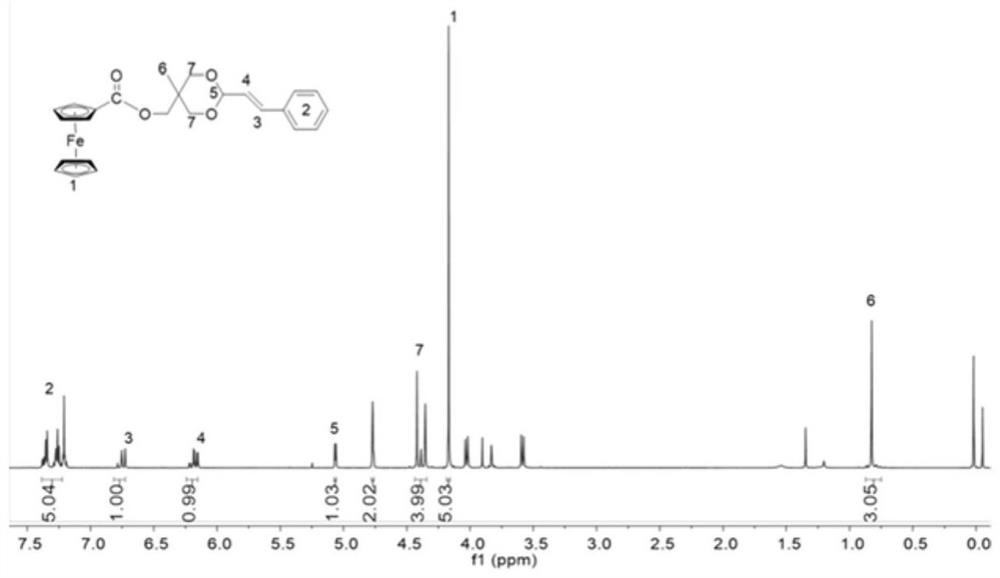
[0043] At 0°C, the excess Fc-COOH (460.088mg, 2mmol) was completely dissolved in 3.5mL of dry anhydrous dichloromethane, EDC (460.056mg, 2.4mmol) was added and stirred for 15min, then CA-OH (515.449 mg, 2.2mmol) was completely dissolved in 1mL of dry anhydrous dichloromethane, then it was added dropwise to the solution of ferrocene in dichloromethane while stirring, after stirring for 15min, DMAP (48.868mg, 0.4mmol) was added, Then react at room temperature for 24h. After the reaction, with saturated NaHCO 3 The aqueous solution was repeatedly extracted and washed 3 times, and the collected organic phase was dried over anhydrous sodium sulfate and concentrated to dryness. Finally, the crude product was purified by a silica gel column (n-hexane:ethyl acetate=6:1) to obtain Fc-CA as an orange-yellow solid.
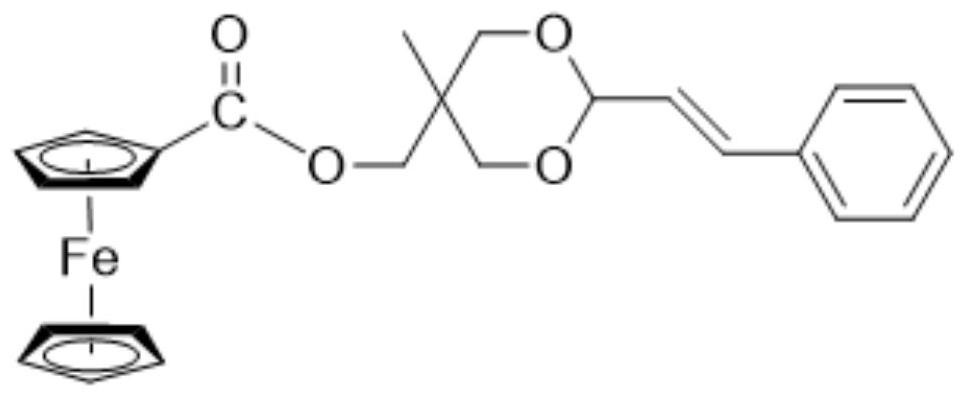
[0044] see figure 1 , figure 1 It is the structural formula of the Fc-CA molecule of the present invention. It can be seen from the figure that the ferrocenecarboxylic a...
Embodiment 2
[0051] At 0°C, the excess Fc-COOH (462.088 mg, 2 mmol) was completely dissolved in 3.5 mL of dry anhydrous dichloromethane, EDC (460.056 mg, 2.4 mmol) was added and stirred for 15 min, then CA-OH (515.852 mg, 2.2mmol) was completely dissolved in 1mL of dry anhydrous dichloromethane, then it was added dropwise to the solution of ferrocene in dichloromethane while stirring, after stirring for 15min, DMAP (48.459mg, 0.4mmol) was added, Then react at room temperature for 24h. After the reaction, with saturated NaHCO 3 The aqueous solution was repeatedly extracted and washed 3 times, and the collected organic phase was dried over anhydrous sodium sulfate and concentrated to dryness. Finally, the crude product was purified by a silica gel column (n-hexane:ethyl acetate=6:1) to obtain Fc-CA as an orange-yellow solid.
Embodiment 3
[0053] At 0°C, the excess Fc-COOH (462.786mg, 2mmol) was completely dissolved in 3.5mL of dry anhydrous dichloromethane, EDC (462.176mg, 2.2mmol) was added and stirred for 15min, then CA-OH (515.449 mg, 2.2mmol) was completely dissolved in 1mL of dry anhydrous dichloromethane, then it was added dropwise to the solution of ferrocene in dichloromethane while stirring, after stirring for 15min, DMAP (49.846mg, 0.4mmol) was added, Then react at room temperature for 24h. After the reaction, with saturated NaHCO 3 The aqueous solution was repeatedly extracted and washed 3 times, and the collected organic phase was dried over anhydrous sodium sulfate and concentrated to dryness. Finally, the crude product was purified by a silica gel column (n-hexane:ethyl acetate=6:1) to obtain Fc-CA as an orange-yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com