Lithium ion battery electrolyte and preparation method and application thereof
A lithium-ion battery and electrolyte technology, applied in the field of lithium-ion batteries, can solve the problems of lowering thermal decomposition temperature, increasing internal resistance, poor high-temperature performance of lithium-ion batteries, etc., and achieving the effect of suppressing the combustion chain reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
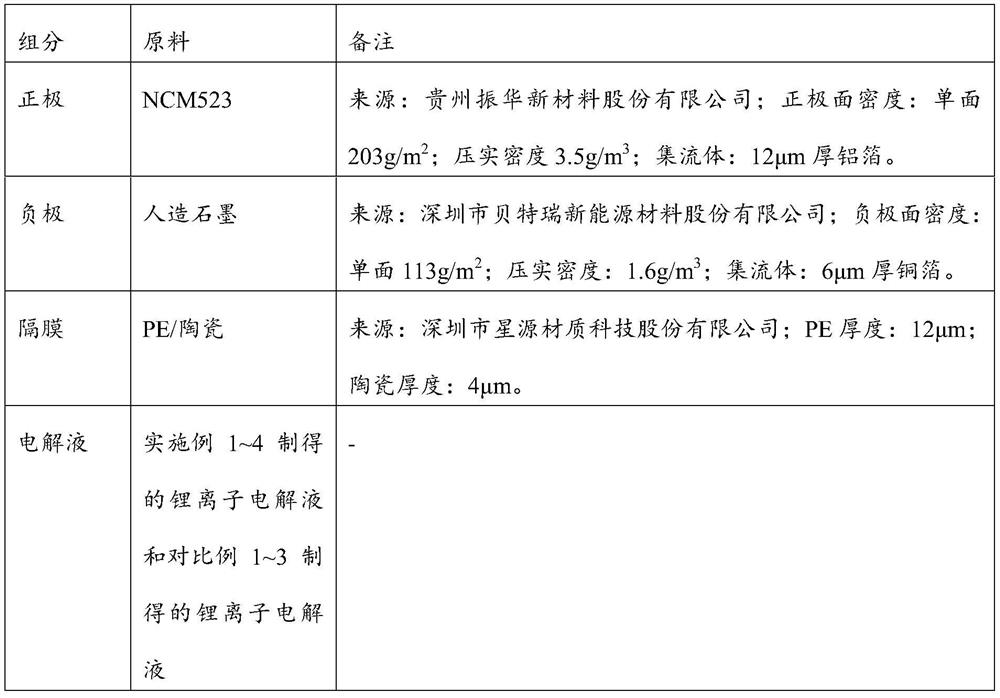
[0071] This embodiment provides a lithium-ion battery electrolyte and a preparation method thereof.
[0072] The lithium ion battery electrolyte of the present embodiment is made up of the preparation raw material of following mass fraction:
[0073] Lithium salt: lithium trifluoromethanesulfonimide;
[0074] Solvent: the mass ratio of dimethyl carbonate, diethyl carbonate and ethylene carbonate is 1:1:1;
[0075] Additives: dimethyl methyl phosphate, bis(2,2,2-trifluoroethyl) carbonate and diphenyldimethoxysilane;
[0076] The molar concentration of lithium salt in the electrolyte is 1mol / L;
[0077] The mass fraction of dimethyl methyl phosphate in the electrolyte is 1%;
[0078] The mass fraction of bis(2,2,2-trifluoroethyl) carbonate in the electrolyte is 2%;
[0079] The mass fraction of diphenyldimethoxysilane in the electrolyte is 0.2%;
[0080] Solvent balance.
[0081] This embodiment also provides a preparation method for the electrolyte, comprising the followi...
Embodiment 2
[0093] This embodiment is a lithium-ion battery electrolyte and a preparation method thereof.
[0094] The lithium ion battery electrolyte of the present embodiment is made up of the preparation raw material of following mass fraction:
[0095] Lithium salt: lithium trifluoromethanesulfonimide;
[0096] Solvent: the mass ratio of dimethyl carbonate, diethyl carbonate and ethylene carbonate is 1:1:1;
[0097] Additives: dimethyl methyl phosphate, bis(2,2,2-trifluoroethyl) carbonate and diphenyldimethoxysilane;
[0098] The molar concentration of lithium salt in the electrolyte is 1mol / L;
[0099] The mass fraction of dimethyl methyl phosphate in the electrolyte is 2%;
[0100] The mass fraction of bis(2,2,2-trifluoroethyl) carbonate in the electrolyte is 1.8%;
[0101] The mass fraction of diphenyldimethoxysilane in the electrolyte is 0.4%;
[0102] Solvent balance.
[0103] The preparation method of this embodiment is the same as in Example 1.
Embodiment 3
[0105] This embodiment is a lithium-ion battery electrolyte and a preparation method thereof.
[0106] The lithium ion battery electrolyte of the present embodiment is made up of the preparation raw material of following mass fraction:
[0107] Lithium salt: lithium trifluoromethanesulfonimide;
[0108] Solvent: the mass ratio of dimethyl carbonate, diethyl carbonate and ethylene carbonate is 1:1:1;
[0109] Additives: dimethyl methyl phosphate, bis(2,2,2-trifluoroethyl) carbonate and diphenyldimethoxysilane;
[0110] The molar concentration of lithium salt in the electrolyte is 1mol / L;
[0111] The mass fraction of dimethyl methyl phosphate in the electrolyte is 3%;
[0112] The mass fraction of bis(2,2,2-trifluoroethyl) carbonate in the electrolyte is 1.5%;
[0113] The mass fraction of diphenyldimethoxysilane in the electrolyte is 0.4%;
[0114] Solvent balance.
[0115] The preparation method of this embodiment is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com