An analytical method for detecting related substances of gefitinib
An analytical method and a chromatographic technique, which are applied in the field of analysis of the detection of gefitinib-related substances, can solve the problems of complex preparation process, inability to separate, and difficult separation of gefitinib, and achieve good separation and low detection limit , good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
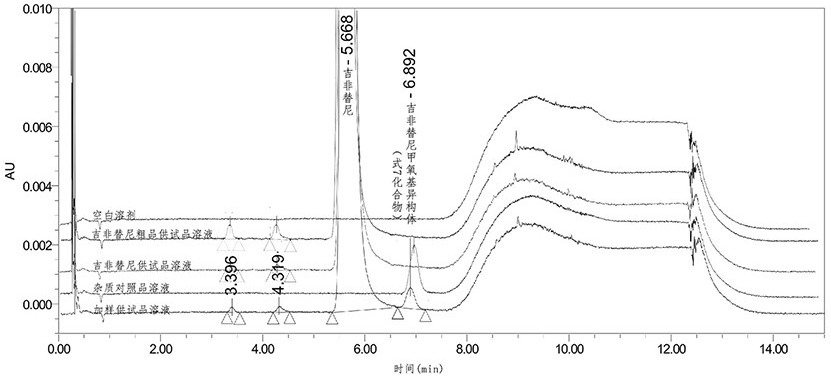
[0071] Example 1 Exclusive detection method for compound of formula 7 in gefitinib.
[0073] Mobile phase B: Weigh 1.54 g of ammonium acetate, add 1 L of methanol, and ultrasonically mix to obtain a 20 mmol / L ammonium acetate methanol solution as mobile phase B.
[0074] Gefitinib crude product test solution: Weigh 10 mg of gefitinib crude product, put it in a 10 mL measuring bottle, add methanol to dissolve and dilute to the scale, mix well, and obtain.
[0075] Gefitinib test solution: Weigh 10mg of Gefitinib, put it in a 10mL measuring bottle, add methanol to dissolve and dilute to the mark, mix well, and obtain.
[0076] Impurity stock solution: Weigh 25mg of the compound of formula 7, put it in a 25mL measuring bottle, add methanol to dissolve and dilute to the mark, accurately measure 1mL, put it in a 100mL measuring bottle, dilute to the mark with methanol, and mix well to get ready.
[0077] Impurity reference substance solution: Accuratel...
Embodiment 2
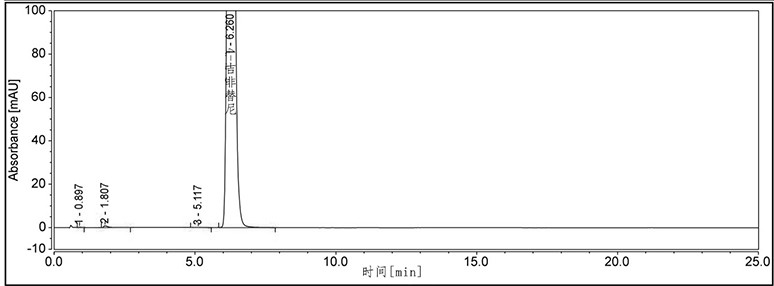
[0086] Example 2 Quantitation limit and detection limit test of the compound of formula 7.
[0088] Mobile phase B: Weigh 1.54 g of ammonium acetate, add 1 L of methanol, and ultrasonically mix to obtain a 20 mmol / L ammonium acetate methanol solution as mobile phase B.
[0089] Detection limit detection solution: Take 1mL of the impurity stock solution in the specificity test, put it in a 20mL measuring bottle, dilute to the mark with methanol, mix well, and get ready.
[0090] Quantitation limit detection solution: Take 2mL of the impurity stock solution in the specificity test, put it in a 10mL measuring bottle, dilute with methanol to the mark, mix well, and get ready.
[0091] According to the test of supercritical fluid chromatography (Chinese Pharmacopoeia 2015 Edition Sibu General Rules 0531), use ethylene bridge hybrid particles as filler (recommended use: Waters Torus 2-PIC (3.0×100mm, 1.7μm)); 2 It is the mobile phase A, 20mmol / L ammoniu...
Embodiment 3
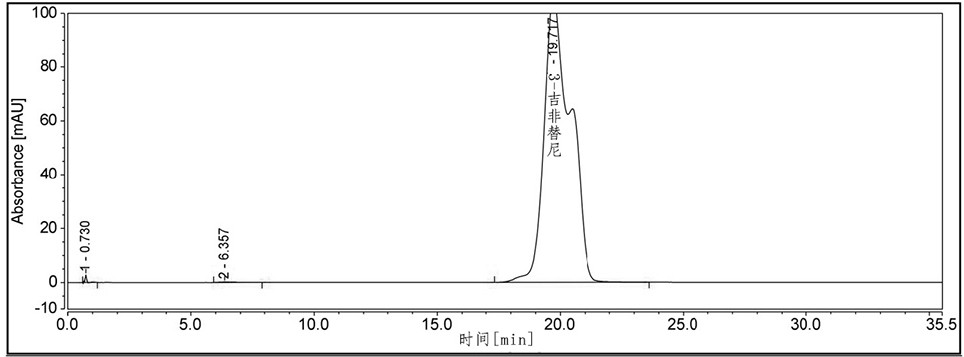
[0098] Example 3 The compound of formula 7 in gefitinib was separated and detected by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com