Application of Tanreqing in preparation of anti-influenza virus medicine
An anti-influenza virus, Tanreqing technology, applied in antiviral agents, drug combinations, pharmaceutical formulas, etc., can solve the problems of anti-influenza virus that have not yet been prepared by Tanreqing preparations, and achieve the goal of inhibiting lung pathological damage and slowing down death Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
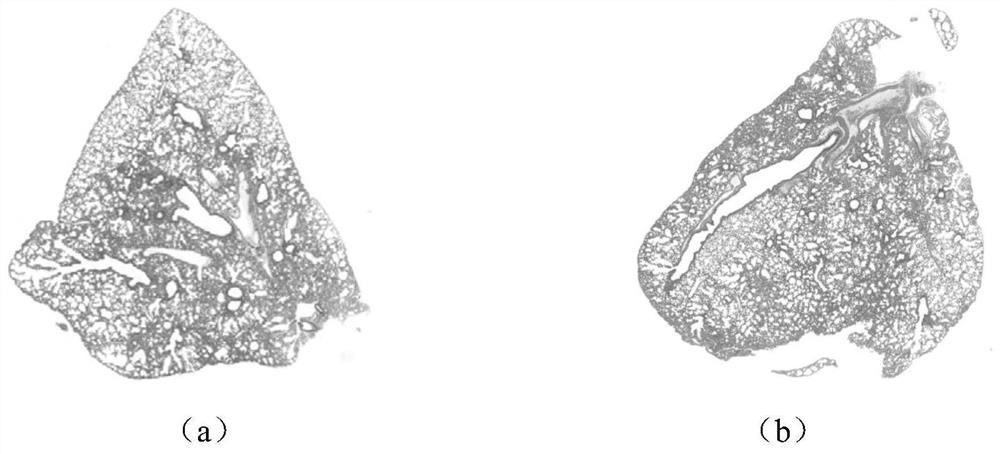
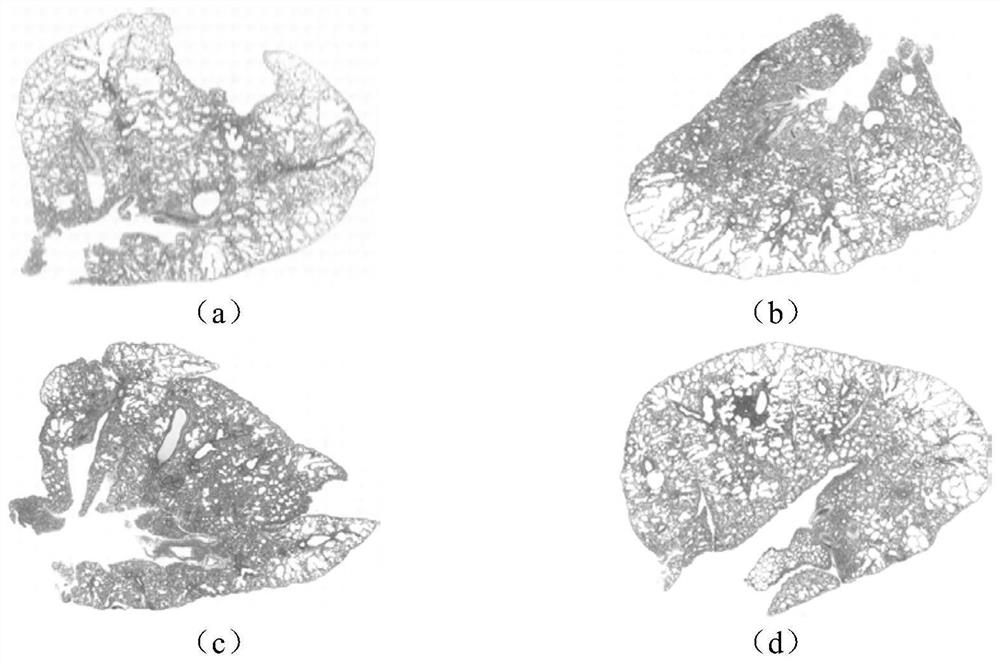
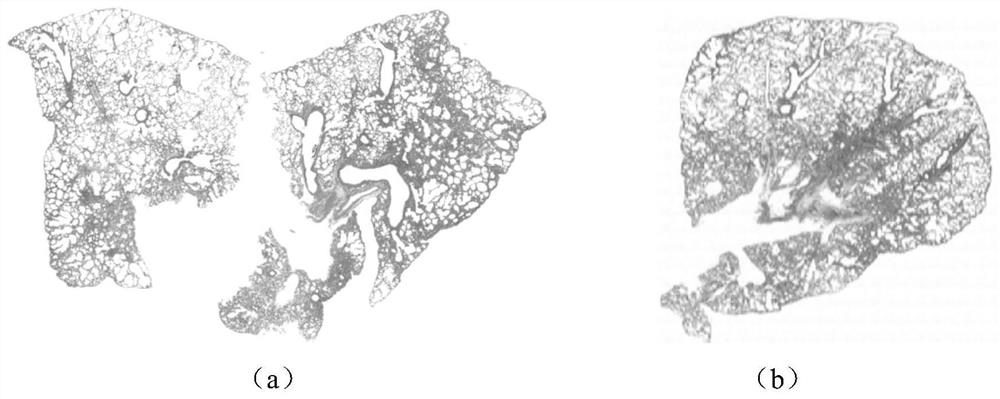
[0033] In order to evaluate the therapeutic effects of Tanreqing Capsules and Tanreqing Injection on influenza virus-induced diseases, the following experiments are now carried out:
[0034] (1) Materials and instruments
[0035] 1.1 Materials
[0036] 1.1.1 Reagent
[0037] Tanreqing Capsules, batch number: 2003108; production date: 20200319; concentration: 1410mg / kg, 470mg / kg, 160mg / kg;
[0038] Tanreqing injection, batch number: 2003206; production date: 20200306; concentration: 7.8mg / kg, 2.6mg / kg, 0.87mg / kg;
[0039] 1.1.2 Experimental animals
[0040] Balb / C mice, grade: SPF grade; age: 6-8 weeks; source: Shanghai Lingchang Biotechnology Co., Ltd.; certificate: 2013001832987;
[0041] 1.1.3 Types of viruses
[0042] Influenza A H1N1-FM1 / 47 murine lung-adapted strain, source: ATCC;
[0043] 1.2 Instruments
[0044] Ultra-low temperature refrigerator (Thermo), IVC system (Jiangsu Fengshi);
[0045] (2) Experimental process
[0046] 2.1 Experimental drug preparation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com