Dichloro-substituted benzothiazole disperse dye with high alkali resistance and high oxygen bleaching resistance
A technology of benzothiazoles and disperse dyes, applied in azo dyes, organic dyes, monoazo dyes, etc., can solve the problem that the relationship between dye molecular structure and alkali resistance and oxidation resistance has not yet formed a system, and achieve Good social and economic benefits, high brightness, and the effect of simplifying the processing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A kind of benzothiazole disperse dye shown in the following structural formula,
[0046]
[0047]Its synthetic route is:
[0048]
[0049] Its preparation method comprises the following steps:
[0050] (1) Diazo reaction: Take 2-amino-5,6-dichlorobenzothiazole (0.04mol) and join in a single-necked flask, add 20.00ml of water and 15.6g of 98% sulfuric acid solution, cool to 0°C, and Add an appropriate amount (12.7g) of nitrosylsulfuric acid under stirring (300-500rpm). After stirring evenly, it turns blue with starch-potassium iodide test paper to ensure that the nitrosylsulfuric acid is excessive. Continue to react for 3 hours. After the reaction is completed, add 3.88g of amino Sulphonic acid eliminates excess nitrosyl sulfuric acid, and continues to stir at 0°C for 15 minutes to obtain diazonium solution;
[0051] (2) Coupling reaction: add 60mL water, 2mL H 2 SO 4 and the coupling component (N-ethyl-N-p-tolylaniline (0.1mol)), cooled to 8°C to obtain the co...
Embodiment 2
[0056] A kind of benzothiazole disperse dye shown in the following structural formula,
[0057]
[0058] Its synthetic route is:
[0059]
[0060] Its preparation method comprises the following steps:
[0061] (1) Diazonium reaction: adjust the "2-amino-5,6-dichlorobenzothiazole" in step (1) of Example 1 to "2-amino-6-chlorobenzothiazole", and other examples Step (1) of 1 is consistent;
[0062] (2) Coupling reaction: consistent with step (2) of Example 1;
[0063] (3) Dissolve the filter cake obtained in step (2) in 95% ethanol solution (the ethanol solution can dissolve the filter cake), reflux at 70° C. for 2 hours, recrystallize after cooling, and obtain benzo Thiazole disperse dyes.
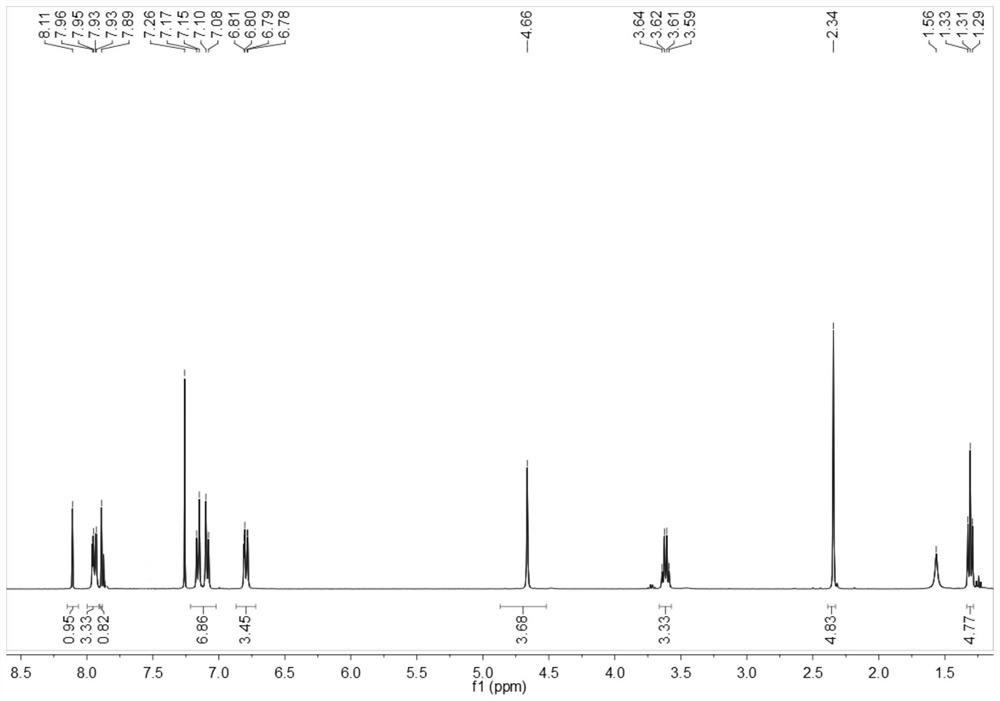
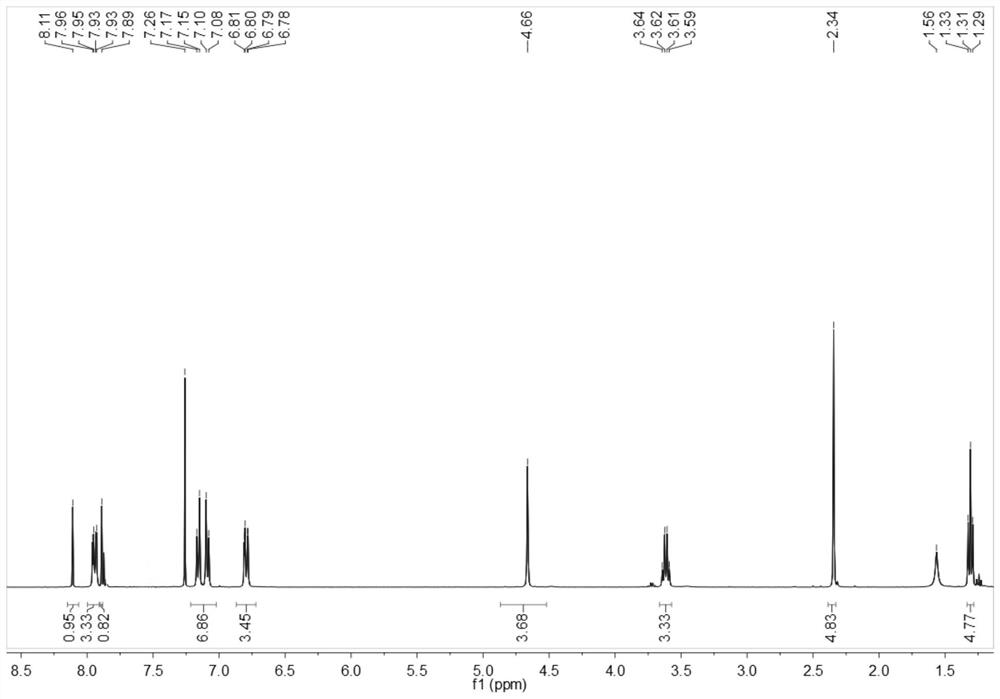
[0064] The obtained benzothiazole disperse dyes are characterized by structure, and the result of H-NMR spectrum ( figure 2 )for:
[0065] 1 H NMR (400MHz, CDCl 3 )δ8.11(s,1H,Ar-H,1),7.96-7.93(dd,2H,Ar-H,3,3'),7.89(s,1H,Ar-H,2),7.26(solvent peak), 7.17-7.08(dd,4H,Ar-H,8,9,10,...
Embodiment 3
[0067] A kind of benzothiazole disperse dye shown in the following structural formula,
[0068]
[0069] Its synthetic route is:
[0070]
[0071] Its preparation method comprises the following steps:
[0072] (1) Diazo reaction: adjust "2-amino-5,6-dichlorobenzothiazole" in step (1) of Example 1 to "2-amino-6-nitrobenzothiazole", and implement Step (1) of Example 1 remains the same;
[0073] (2) Coupling reaction: consistent with step (2) of Example 1;
[0074] (3) Dissolve the filter cake obtained in step (2) in 95% ethanol solution (the ethanol solution can dissolve the filter cake), reflux at 70° C. for 2 hours, recrystallize after cooling, and obtain benzo Thiazole disperse dyes.
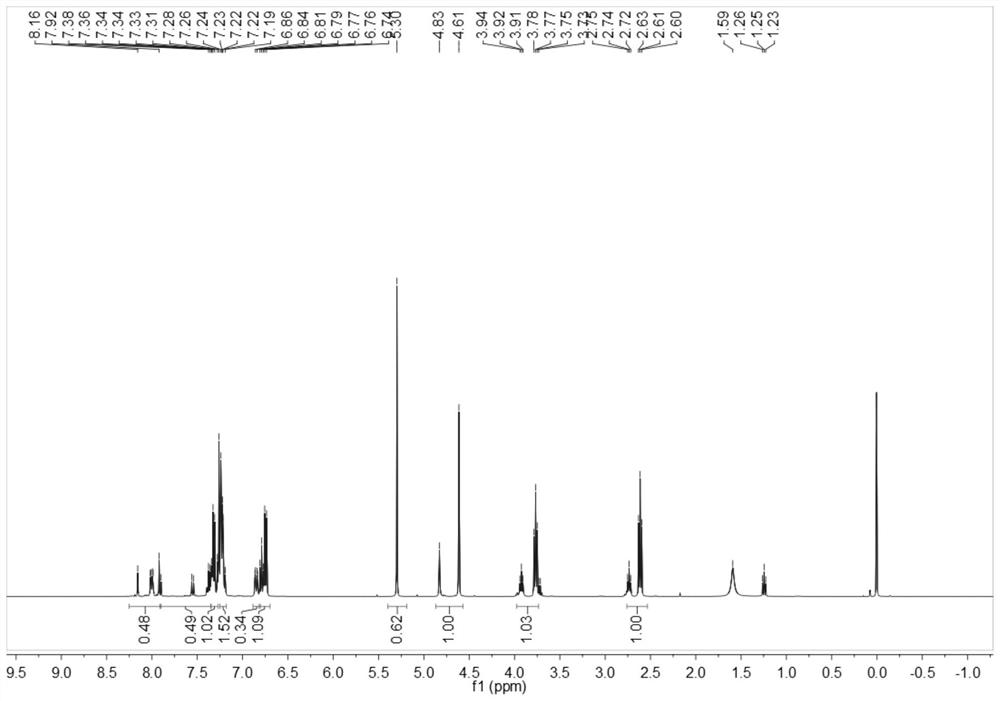
[0075] The obtained benzothiazole disperse dyes are characterized by structure, and the result of H-NMR spectrum ( image 3 )for:
[0076] 1 H NMR (500MHz, CDCl 3 )δ8.08-7.99(d,1H,Ar-H,1),7.96-7.85(d,1H,Ar-H,2)7.85-7.49(dd,1H,Ar-H,3),7.26(solvent peak), 7.17-7.07(dd,4H,Ar-H,10,11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com