Preparation method of CSF-IR inhibitor
A reagent and catalyst technology, applied in the field of preparation of CSF-IR inhibitors, can solve the problems of unsuitability for industrial scale-up production, high preparation cycle and cost, insufficient substitution reaction yield, etc., to improve atom economy and reduce reaction difficulty. , the effect of shortening the synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
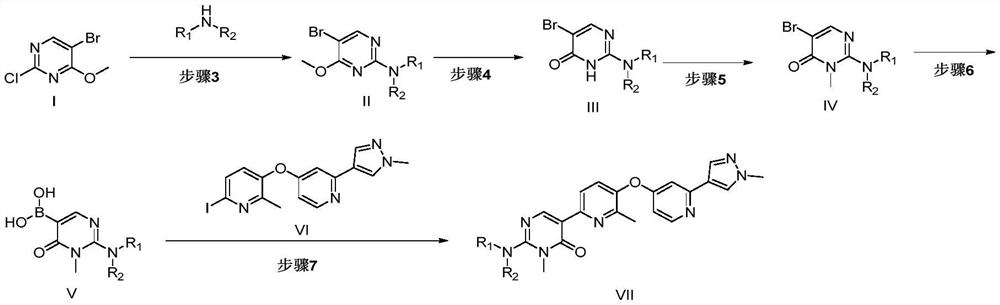
[0047] In order to solve the above-mentioned technical problems, the first aspect of the present invention provides a preparation method of compound VII, which is characterized in that, comprising the following steps:
[0048] Step 3: Compound I is reacted with an organic amine under the action of a base to obtain Compound II;
[0049] Step 4: Compound II is demethylated and dearomatized to obtain Compound III;
[0050] Step 5: Compound III reacts with a methylating reagent under alkaline conditions to obtain Compound IV;
[0051] Step 6: Compound IV is reacted with a borate ester compound in the presence of a lithium reagent to obtain Compound V;
[0052] Step 7: Coupling reaction between compound V and compound VI to obtain VII;
[0053]
[0054] Among them, R 1 and R 2 identically or differently selected from hydrogen, C 1~6 saturated or unsaturated alkyl groups.
[0055] In some preferred embodiments, the R 1 and R 2 Respectively selected from hydrogen, isopropy...
Embodiment 1
[0120] Example 1, Preparation of Compound VI
[0121]
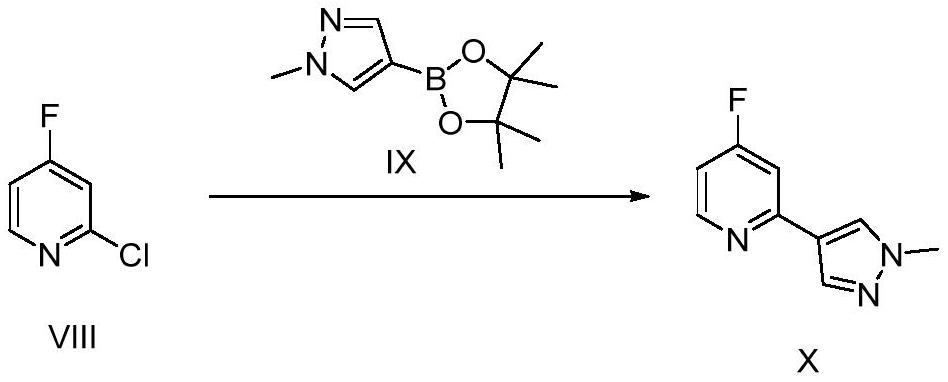
[0122] Compound VIII (100g, 760mmol), compound IX (174g, 836mmol), K 2 CO 3 (316g, 2280mmol) mixed with dioxane / water (1.2L) at a volume ratio of 5:1, replaced by argon, and added 3 ) 4 (10g), replaced by inert gas, and heated overnight at 90°C; after the reaction, filter, add water to the filtrate, extract with EA (2L×3), add anhydrous sodium sulfate to the combined organic phase to dry, filter, and concentrate the filtrate , the resulting crude product was purified by flash column chromatography (PE / EA=2:1) to obtain pure compound X (122 g, 90%).
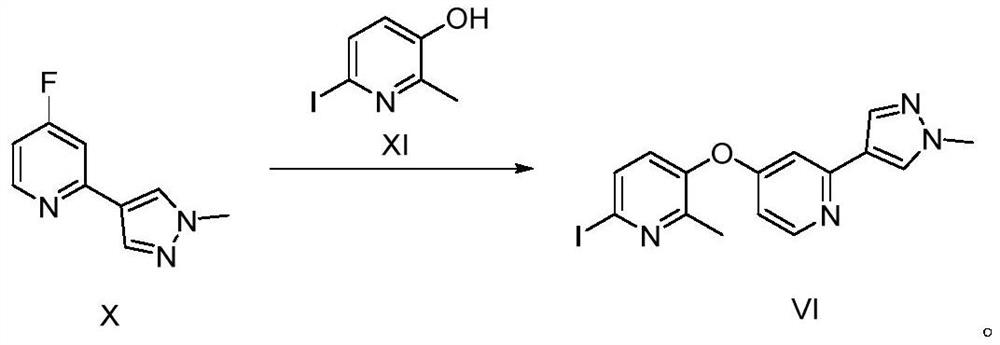
[0123] Compound XI (99g, 423mmol) and K 2 CO 3 (39g, 282.2mmol) was dispersed in DMA (500mL), stirred at room temperature for 30min, compound X (50g, 282.2mmol) was added, and heated at 115°C overnight; after the reaction, cooled to room temperature, added H 2 O (1.5L), extracted with EA (3L×3), the organic phases were combined, washed with brine (3L), separated, ...
Embodiment 2
[0125] Example 2, Preparation of Compound VI
[0126]
[0127] Compound VIII (100g, 760mmol), compound IX (126.5g, 608mmol), K 2 CO 3 (210g, 1520mmol) mixed with dioxane / water (1L) with a volume ratio of 5:1, replaced with argon, and added Pd(PPh 3 ) 4 (8.2g), replaced by inert gas, and heated overnight at 90°C; after the reaction, filter, add water to the filtrate, extract with EA (2L×3), add anhydrous sodium sulfate to the combined organic phase, filter, concentrate The filtrate was purified by flash column chromatography (PE / EA=2:1) to obtain pure compound X (117 g, 87%).
[0128] Compound XI (66.3g, 282.2mmol) and K 2 CO 3 (31.2g, 225.76mmol) was dispersed in DMA (450mL), stirred at room temperature for 30min, compound X (50g, 282.2mmol) was added, and heated at 115°C overnight; after the reaction, cooled to room temperature, added H 2 O (1L), extracted with EA (3L×3), combined the organic phases, washed with brine (2.5L), separated the layers, added anhydrous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com