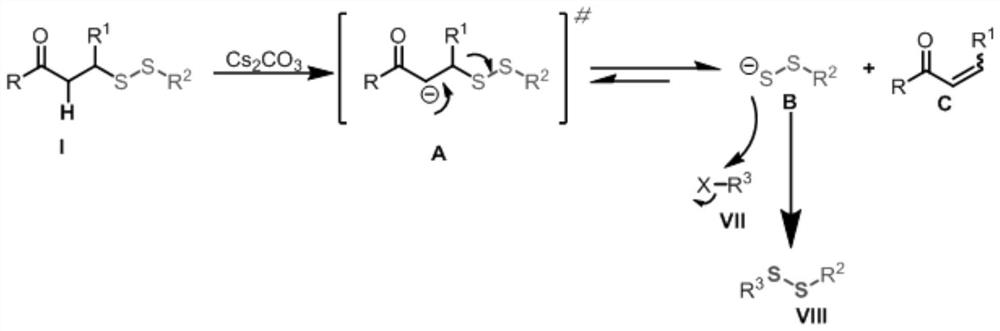
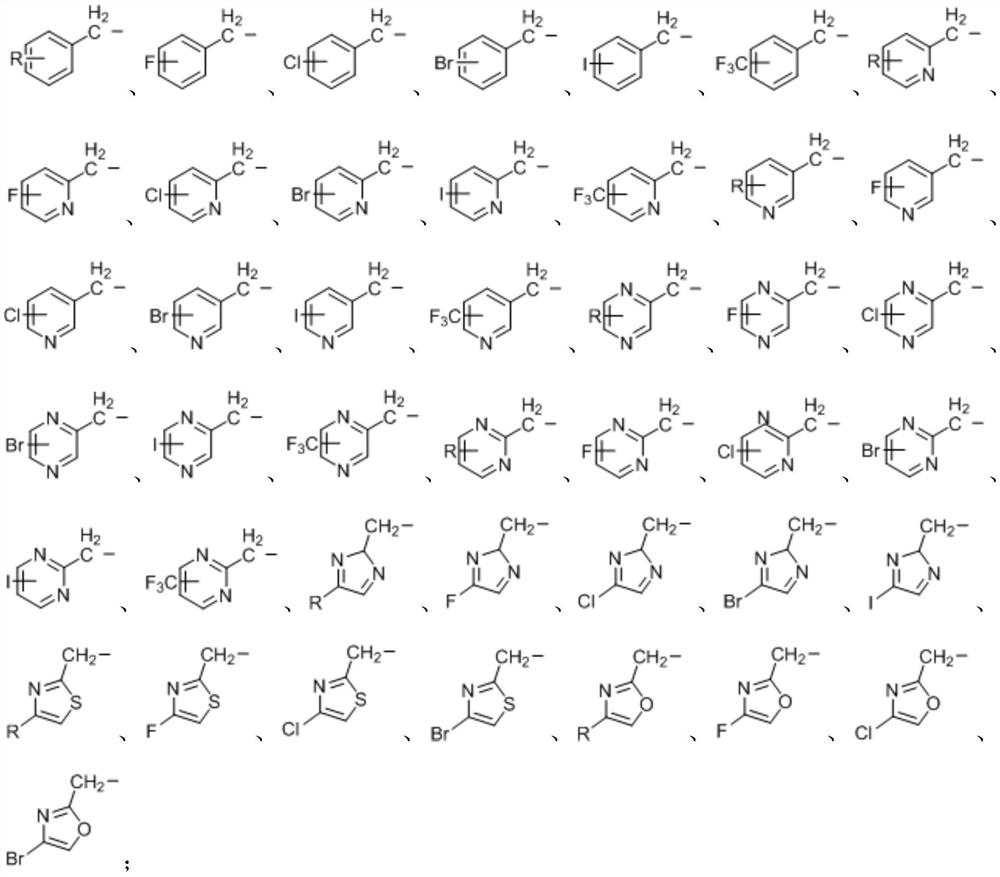
Application of novel persulfide reagent in synthesis of asymmetric persulfides
A persulfide, asymmetric technology, used in the application field of new persulfide reagents in the synthesis of asymmetric persulfides, can solve the unpleasant odor and high toxicity of thiols, poor homocoupling by-products, and suitable substrates. narrow range and other problems, to avoid homocoupling by-products, wide application range of substrates, and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Step 1: 1-Penten 3-one (2.5 g, 30 mmol, 1.0 equiv) and thioacetic acid (2.9 g, 39 mmol, 1.3 equiv) were added to EA (20 mL) at room temperature. And stir for 6h. Afterwards, the reaction was quenched with ice water and extracted into EA (3x20 mL). The combined organics were dried over sodium sulfate, filtered and concentrated in vacuo to give a clear colorless oil which was used without further purification. MeOH (10 mL) was added to the concentrated solution from the previous step. Then add H 2 SO 4 (2mol / L) solution to a pH range of 1-2, and the mixture was stirred at 85°C for 12h. Water (15 mL) was then added. After the organic layer was separated, the aqueous layer was extracted with 20 mL of ethyl acetate, the extract was combined with the organic layer, and washed successively with 5% aqueous sodium carbonate and saturated aqueous sodium chloride. The organic layer was dried over magnesium sulfate, filtered, and the organic solvent was distilled ...
Embodiment 2
[0047] Preparation of 1-(tert-butyldisulfanyl)pentan-3-one, the reaction scheme is as follows:
[0048]
[0049] 1-Mercapto-3-pentanone (5.9 g, 50 mmol, 1.0 equiv) was dissolved in 95% ethanol (40 mL), then tert-butylmercaptan (450 g, 500 mol, 10 equiv) was added. The mixture solution was cooled to 0° C., and an ethanol solution of iodine (10 g of iodine in 12 mL of ethanol) was added dropwise until the color of the reaction changed from colorless to red. After 12 h, saturated aqueous sodium bicarbonate solution was added until pH>7. The solution was concentrated in vacuo. The disulfide was extracted with ethyl acetate, and the organic layer was washed with 10% sodium bisulfite and brine. Finally, the organic layer was dried over sodium sulfate, filtered, concentrated and purified by flash chromatography (hexanes) to give the pure product as a brown liquid in 43% yield.
[0050] The proton nuclear magnetic resonance spectrum analysis result is as follows:
[0051] 1 H ...
Embodiment 3
[0059] To prepare 4-(tert-butyldisulfanyl)pentan-2-one, the reaction scheme is as follows:
[0060]
[0061] 4-Mercapto-2-pentanone (3.54 g, 30 mmol, 1.0 equiv) was dissolved in 95% ethanol (20 mL), then tert-butylmercaptan (27 g, 300 mmol, 10 equiv) was added. The mixture solution was cooled to 0° C., and an ethanol solution of iodine (10 g of iodine in 12 mL of ethanol) was added dropwise until the color of the reaction changed from colorless to red. After 12 h, saturated aqueous sodium bicarbonate solution was added until pH>7. The solution was concentrated in vacuo. The disulfide was extracted with ethyl acetate, and the organic layer was washed with 10% sodium bisulfite and brine. Finally, the organic layer was dried over sodium sulfate, filtered, concentrated and purified by flash chromatography (hexanes) to give the pure product as a brown liquid in 32% yield.
[0062] The proton nuclear magnetic resonance spectrum analysis result is as follows:
[0063] 1 H NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com