Acetazolamide sustained-release capsule and preparation method thereof
A technology of acetazolamide and sustained-release capsules, which is applied in the field of medicine, can solve problems such as uneven mixing and affecting the release uniformity of unit sustained-release capsules, and achieve the effect of stable release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0078] The prescription composition of the acetazolamide sustained-release capsules (500 mg / capsule) of Example 1-2 is shown in Table 5.
[0079] Table 5 Embodiment 1-2 prescription composition
[0080]
[0081] Preparation:
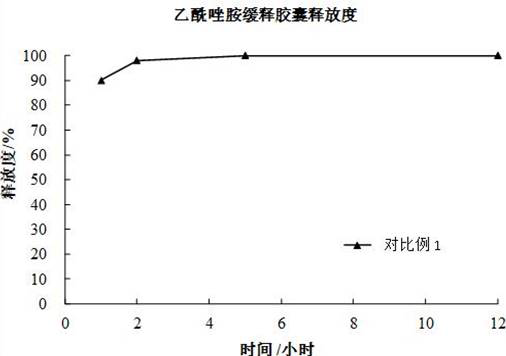
[0082] Step 1-3 and step 5-6 of embodiment 1-2 are identical with comparative example 1;
[0083] Step 4: The melting point of carnauba wax is 80-86°C, and the wet pellets are dried at 85°C for 2 hours.
[0084] Release Determination:
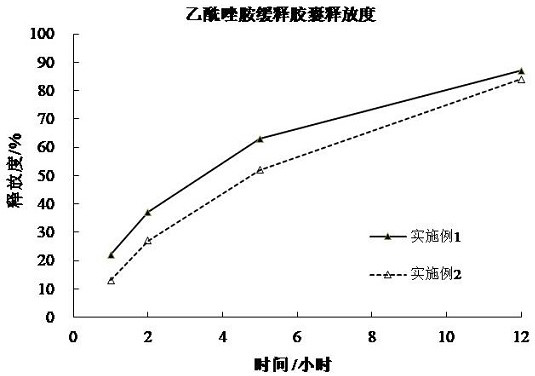
[0085] The release curve of embodiment 1-2 is as figure 2 shown. from figure 2 It can be seen that after the wet pellets of Example 1-2 are heated at a temperature close to the melting point of carnauba wax, even if the prescription amount of carnauba wax is lower than that of Comparative Example 1, it can still provide 12 hours of slow release, indicating that high temperature drying The process has a great influence on the release rate; and the greater the prescription dosage of carnauba wax, the slower the r...
Embodiment 3
[0087] The prescription composition of the acetazolamide sustained-release capsules (500 mg / capsule) of Example 3 is shown in Table 6.
[0088] Table 6 Embodiment 3 prescription composition
[0089]
[0090] Preparation:
[0091] Steps 1-3 and steps 5-6 of embodiment 3 are identical with comparative example 1;
[0092] Step 4: The melting point of glyceryl behenate is 65-77°C, and the wet pellets are dried at 70°C for 2 hours.
[0093] Release Determination:
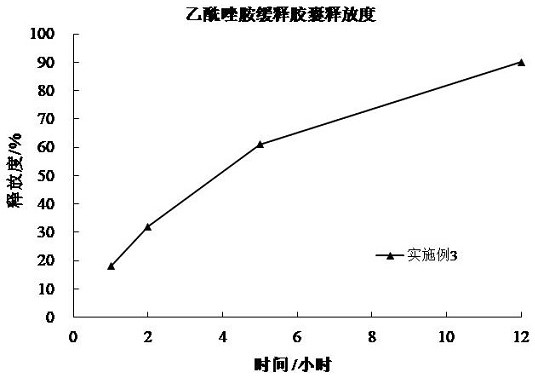
[0094] The release curve of embodiment 3 is as image 3 shown. from image 3 It can be seen that the wet pellets of Example 3 can provide slow release for 12 hours after being heated at a temperature close to the melting point of glyceryl behenate.
[0095] Release Similarity Determination:
[0096] Comparison of release in pH=1.0 hydrochloric acid solution, pH=4.5 acetate buffer, pH=6.8 phosphate buffer and water, using f 2 Similar factor method is to embodiment 1 sustained-release capsule and reference prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com