Application of lapatinib and/or medicinal derivatives thereof in preparation of anti-enterovirus drugs
A technology of lapatinib and enterovirus, applied in the field of application of lapatinib and/or its pharmaceutically acceptable derivatives in the preparation of anti-enterovirus drugs, can solve the problem of limited treatment methods, large individual differences, Difficult to promote and other issues, to achieve strong anti-virus, simple synthesis process, and enhance the effect of cell survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the cytotoxicity detection of lapatinib
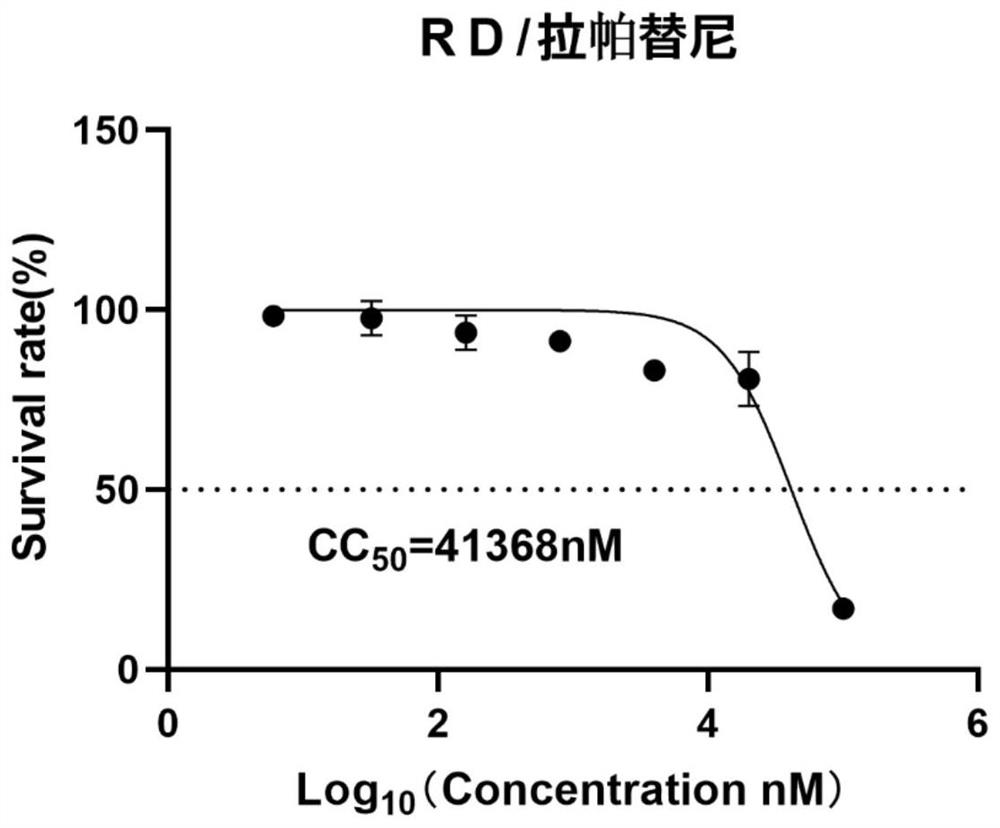
[0043] Cytotoxicity of lapatinib was tested in RD cells. RD cells were seeded in 96-well plates at 37 °C, 5% CO 2 After culturing in the incubator for 12-16 hours, the cell culture medium was discarded, and the cell maintenance medium containing different concentrations of tested lapatinib was added to continue the culture, with 3 replicate wells in each group, and the same amount of PBS was added to the control group. After 48 hours of treatment, stain with MTT and detect OD 492 nm, analysis of cell viability.
[0044] Result analysis: The test results are as follows: figure 1 As shown, the Prism7 software calculates the median toxic concentration (Median cyctoxic concentration, CC 50 ), CC of lapatinib 50 is 41368nM. In subsequent examples, the maximum concentration of lapatinib used is 20000 nM, which is within the safe and non-toxic range.
Embodiment 2
[0045] Example 2, lapatinib inhibits the cytopathic effect of EV71 virus replication
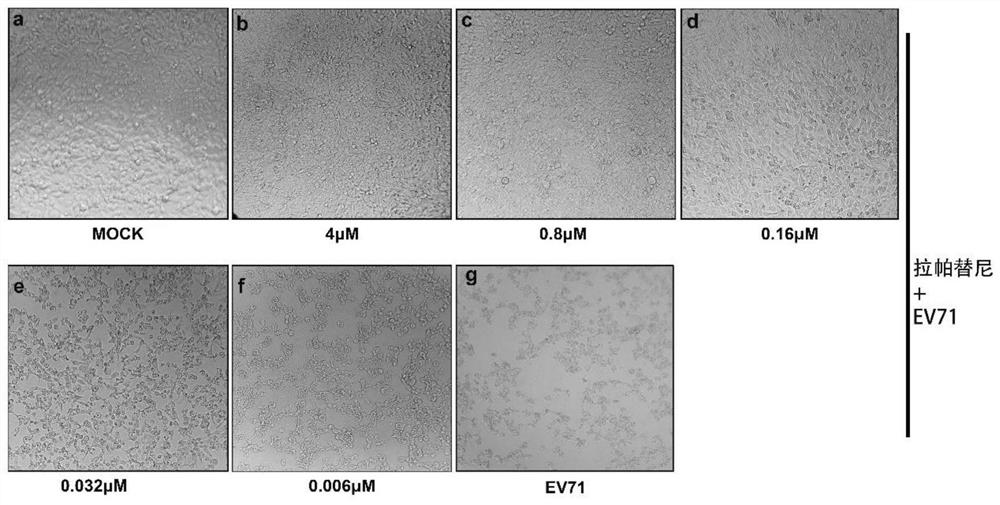
[0046] Plate RD cells on 96-well cell culture plates at 37 °C, 5% CO 2 After culturing in the incubator for 12-16h, 100TCID 50 The cells were infected with the EV71 virus solution for 2 hours, and the cell maintenance solution containing different concentrations of lapatinib was added to continue culturing for about 48 hours, and the same volume of PBS was added to the negative control. When about 90% of the CPE lesions appeared in the virus control wells, the cytopathic effect (CPE) was observed under a microscope.
[0047] Result analysis: The test results are as follows: figure 2 As shown, lapatinib inhibited the CPE effect of RD cells induced by EV71 in a concentration-dependent manner. EV71-infected RD cells became round and detached from the cell wall. Treatment with different concentrations of experimental compounds had a significant inhibitory effect on the lesion effect, effectiv...
Embodiment 3
[0048] Example 3, detection of lapatinib inhibiting EV71 antiviral activity
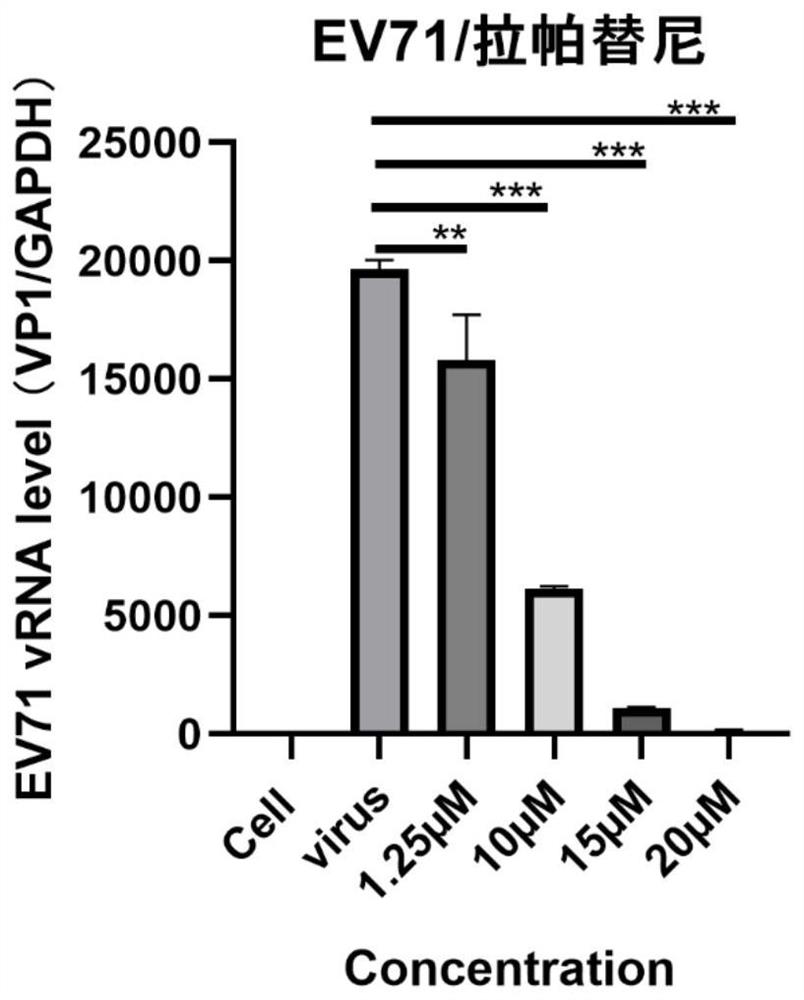
[0049] RD cells were seeded in 24-well cell culture plates at 37°C, 5% CO 2 Cultivate in the incubator for 12-16h, use 100TCID 50 After 2 hours of infection with the EV71 virus liquid, the cell maintenance solution containing different concentrations of experimental lapatinib was used for treatment, and the negative control was added with the same volume of PBS. After 24 hours of treatment, the effect of inhibiting virus proliferation was detected by real-time fluorescent quantitative PCR.
[0050] Result analysis: The test results are as follows: image 3 As shown, under different concentration conditions, the compound lapatinib has a significant inhibitory effect on the replication of EV71. Lapatinib is used as an anti-EV71 virus drug, and when the concentration of lapatinib is 20 μM, the inhibition rate to EV71 virus is 93.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com