Oxaliplatin-containing nano-micelle preparation and medical application thereof
A technology of oxaliplatin and nano micelles, which is applied in the field of pharmaceutical preparations, can solve the problems of significant toxicity, multiple distributions, and lack of specificity, and achieve the effects of improving medicinal effects, simple preparation methods, and prolonging the storage period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] 1. Preparation of oxaliplatin copolymer micelles (taking Pluronic 123 as the hydrophilic segment as an example)
[0025] Dissolve Pluronic 123 (P123), tocopheryl succinate (TOS), and condensing agent 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide in DMSO, and the mixture is continuously stirred After 10 hours, the produced chemical polymer was dialyzed with a dialysis bag with a molecular weight cut-off of 3500, and the product was freeze-dried and sieved to obtain P123-TOS copolymer micelles.
[0026] Dissolve oxaliplatin and sieved P123-TOS copolymer micelles in acetonitrile and stir for 40 minutes, then put the above organic mixture in a dialysis bag, dialyze in distilled water for 12 hours, replace the distilled water every 3 hours, and the dialysis is completed After centrifugation under a high-speed centrifuge, the supernatant was filtered and freeze-dried through a 0.45 μm microporous membrane to obtain oxaliplatin-P123-TOS copolymer micelles
[0027] Two, specif...
Embodiment 1
[0028] Embodiment 1: the formulation containing oxaliplatin nanomicelle
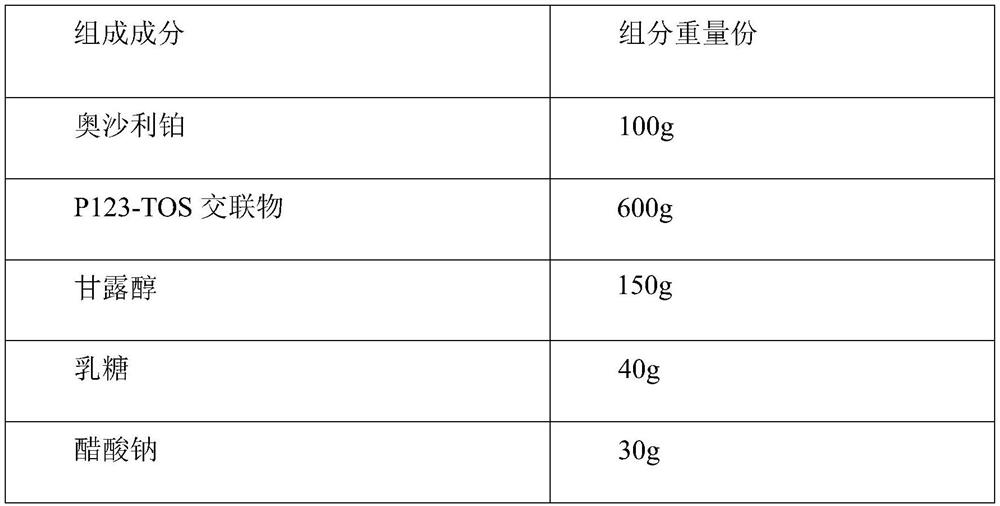
[0029]
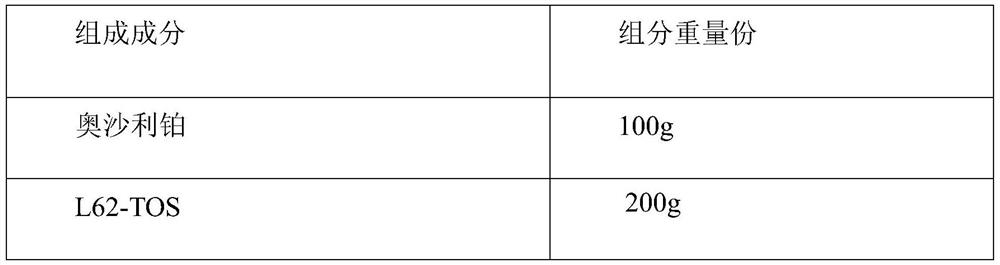
[0030]
[0031] Weigh mannitol, lactose and sodium acetate, add 80% water for injection, dissolve and stir evenly, then add oxaliplatin-P123-TOS copolymer micelles after passing through a 200 mesh sieve, dissolve and stir evenly, and use 0.1 mol / L hydrochloric acid or 0.1mol / L sodium hydroxide solution to adjust the pH to 6.5, after passing the intermediate inspection, add water for injection to make up to full volume. The solution was sent to a sterile room by a peristaltic pump, filtered through a 0.22 μm microporous membrane until clear, and freeze-dried to obtain a freeze-dried powder injection containing oxaliplatin nanomicelles.
Embodiment 2
[0032] Embodiment 2: the formulation containing oxaliplatin nanomicelle
[0033] Composition Component parts by weight Oxaliplatin 100g P85-TOS 1000g Mannitol 180g lactose 50g Sodium acetate 45g Water for Injection Appropriate amount
[0034] Wherein the preparation of P85-TOS is the same as the preparation of the above-mentioned P123-TOS, wherein P85 refers to Pluronic 85
[0035] Weigh mannitol, lactose and sodium acetate, add 80% water for injection, dissolve and stir evenly, then add the oxaliplatin-P85-TOS copolymer micelles passed through a 250 mesh sieve, dissolve and stir evenly, use 0.1 mol / L hydrochloric acid or 0.1mol / L sodium hydroxide solution to adjust the pH to 6.0, after passing the intermediate inspection, add water for injection to make up to full volume. The solution was sent to a sterile room by a peristaltic pump, filtered through a 0.22 μm microporous membrane until clear, and freeze-dried to obtain a f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com