Application of adipose-derived stem cells in localized scleroderma
A technology for localized scleroderma and fat source, applied in skin diseases, medical preparations containing active ingredients, unknown raw materials, etc., can solve the problems of tissue atrophy and adhesion, poor local blood supply, low fat survival rate, etc., to achieve The effect of reducing skin collagen content, reducing symptoms, and improving fat survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Culture and Expansion of Adipose-derived Stem Cells
[0027] Adipose tissue was obtained from the Department of Plastic Surgery, Peking Union Medical College Hospital, and the discarded adipose tissue after liposuction, and informed consent was obtained from the patients.
[0028] 1. Extraction of fat stem cells
[0029] (1) Add 20ml of fat obtained from liposuction into a 50ml centrifuge tube, add D.hangks to 40ml, and centrifuge at 800r for 3min;
[0030] (2) Suck off the liquid at the bottom, transfer the fat to a new centrifuge tube, wash D.hangks twice, and centrifuge at 800r for 3min;
[0031] (3) Add collagenase to the tube, the volume is 1 / 3-1 / 4 of the fat volume, shake at 37°C, 200rpm, 30min;
[0032] (4) After the shaker is taken out, filter with a 100nm filter (wet the filter with Dhangks, which is good for filtering);
[0033] (5) The filtrate was centrifuged at 1500r for 8min;
[0034] (6) After centrifugation, absorb the upper layer of grease...
Embodiment 3
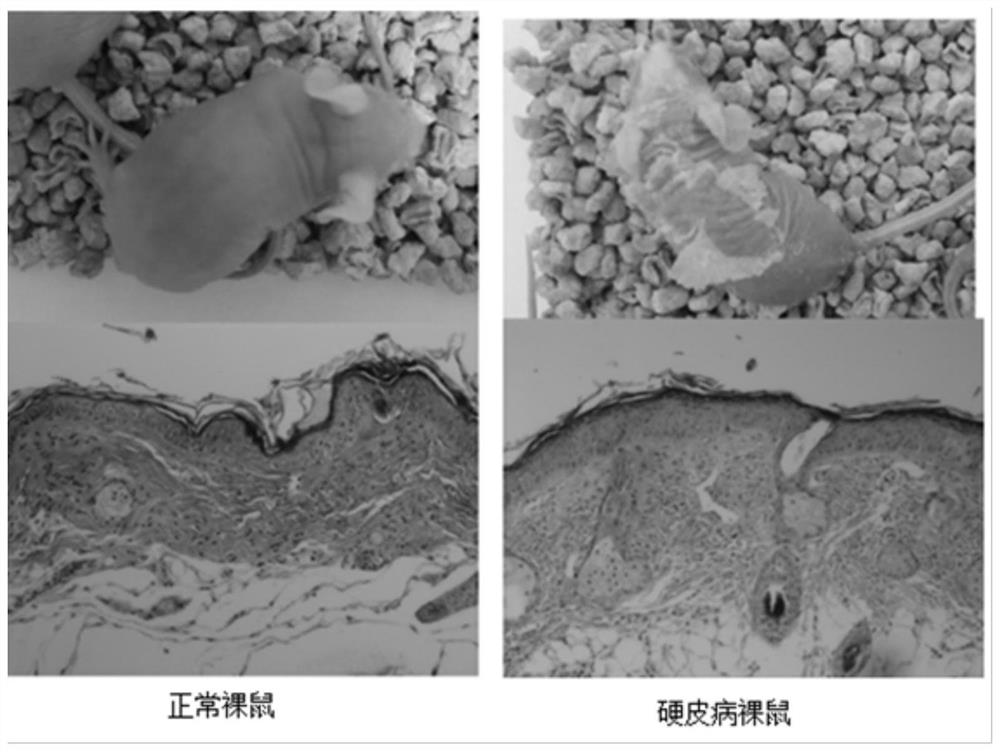
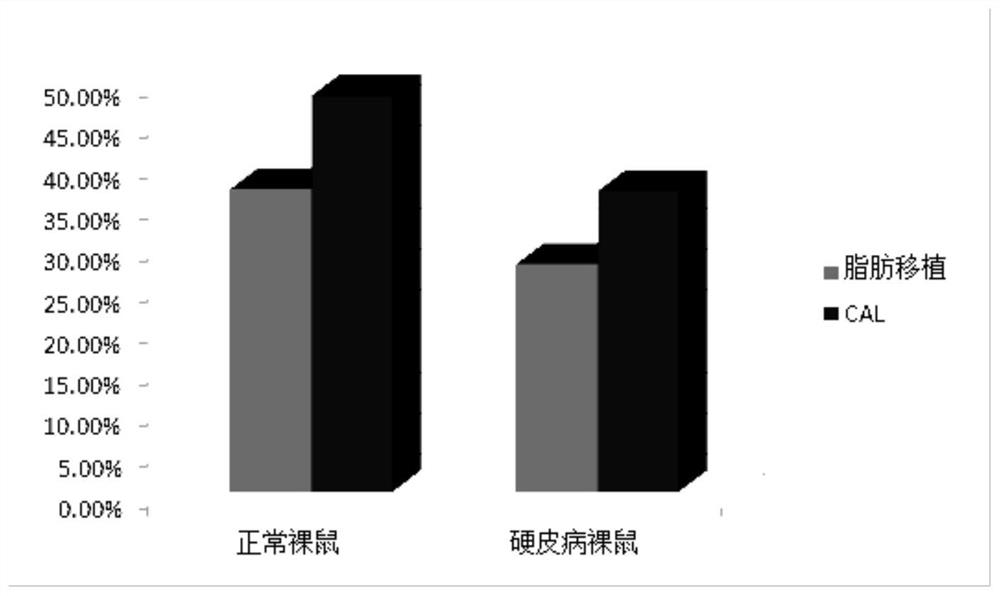
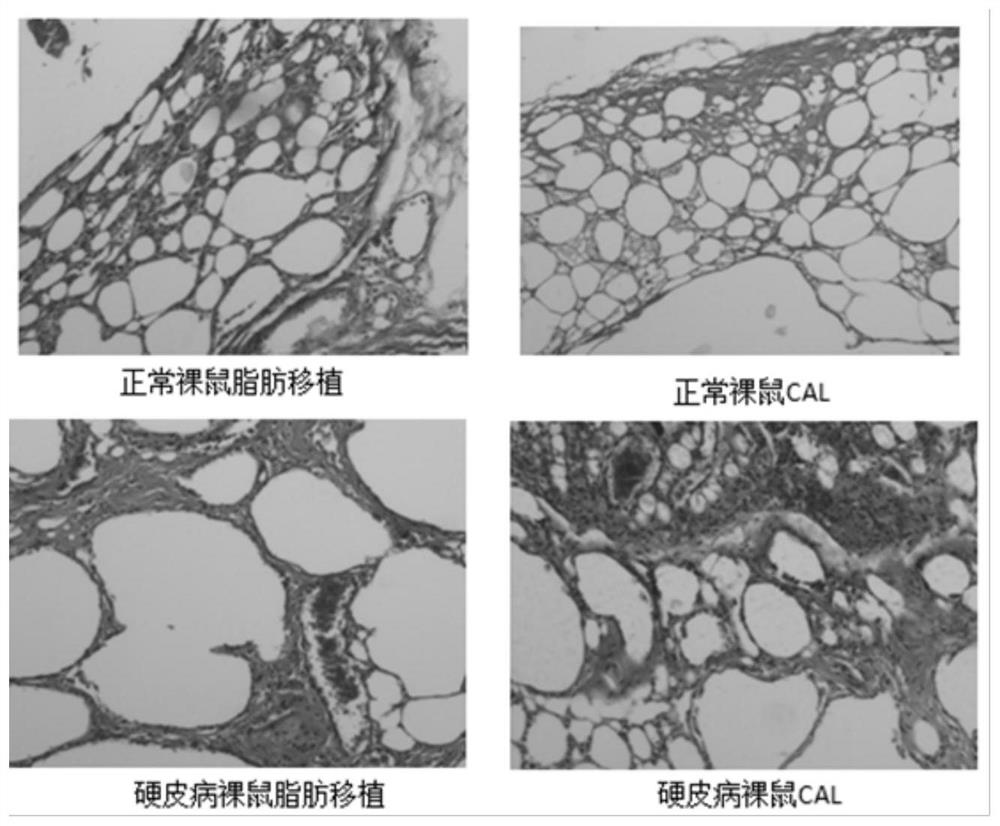
[0051] Example 3 Adipose stem cell-assisted fat transplantation in the treatment of scleroderma nude mouse model
[0052] 1. Modeling of nude mice with bleomycin scleroderma
[0053] The bleomycin powder was diluted to 200ug / ml with phosphate buffered saline (PBS), filtered and sterilized through a 0.22um sterile filter membrane, and stored for future use. In the modeling group, bleomycin dilution was injected subcutaneously on the back of nude mice, and in the control group, PBS was injected subcutaneously on the back of nude mice, once a day, 0.1ml each time, for a total of 4 weeks, and the elasticity and appearance of the back skin were observed. General changes, 2 mice in the pre-experiment modeling group and 2 mice in the control group were killed at 4 weeks, the back skin of the injection area was taken and fixed in tissue fixative, and HE staining and Masson staining were performed respectively. Compared with the mice in the PBS control group, it was confirmed that Whe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com