Method for realizing temporary restorability of dynamically stable gel based on pure chemical reaction
A dynamic and stable technology, applied in the field of temporary repair of kinetically stable gels based on pure chemical reactions, can solve problems such as hindering the development of multifunctional synthetic materials, reducing the repair ability of gels, and affecting the activity of biological substances. High acceptance, avoid brittleness, reduce consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The second aspect of the present invention provides a method for preparing the above-mentioned kinetically stable gel material, comprising the following steps:
[0034] Soaking the gel containing the acylhydrazone bond or the imine bond into the solution formed by mixing the alkaline compound aqueous solution and the antifreezing agent to obtain the gel material.
[0035] Further, the gel containing acylhydrazone bond or imine bond is made of copolymer PAM-co-PDAAM and adipate dihydrazide (ADH) or glutaraldehyde and bovine serum albumin (BSA) at pH 4-5 obtained by cross-linking under conditions.
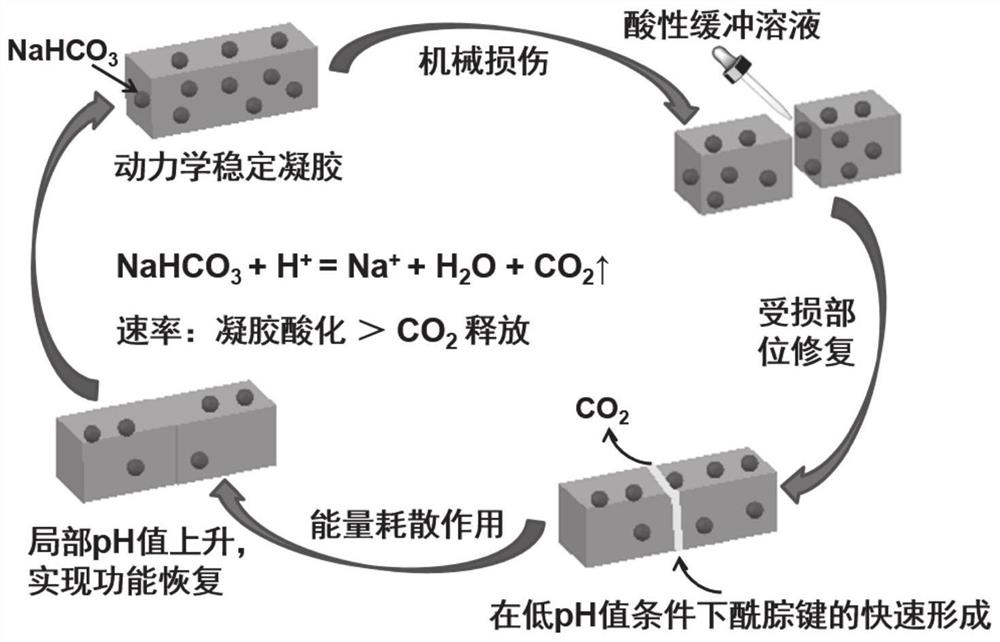
[0036] Kinetically stable gel materials were prepared by partially replacing water molecules in pre-synthesized low-pH pristine hydrogels. During the solvent replacement process, basic chemical molecules are introduced into the gel material not only to inactivate the acylhydrazone bond or imine bond to obtain a kinetically stable material, but also to facilitate the repair of...
Embodiment 1
[0047] The low-pH pristine hydrogel containing acylhydrazone bond obtained by cross-linking PAM-co-PDAAM and ADH at pH 4 was soaked into NaHCO-containing 3 The final gel material was obtained in aqueous ethylene glycol solution. Among them, the concentration of PAM-co-PDAAM is 162mg / mL, the concentration of ADH is 15mg / mL, NaHCO 3 The concentration is 0.03M, and the mass fraction of ethylene glycol is 0-90%.
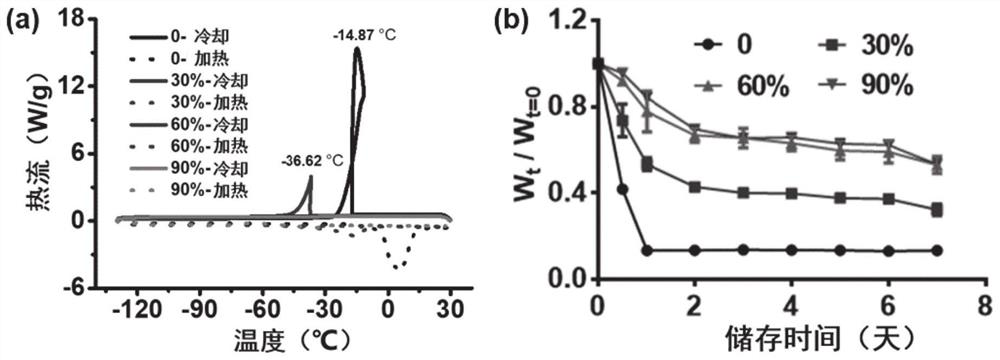
[0048] Such as figure 2 As shown, from the antifreeze and anti-volatility properties of gel materials prepared by adding different contents of ethylene glycol, the addition of 60% and 90% has the best performance.
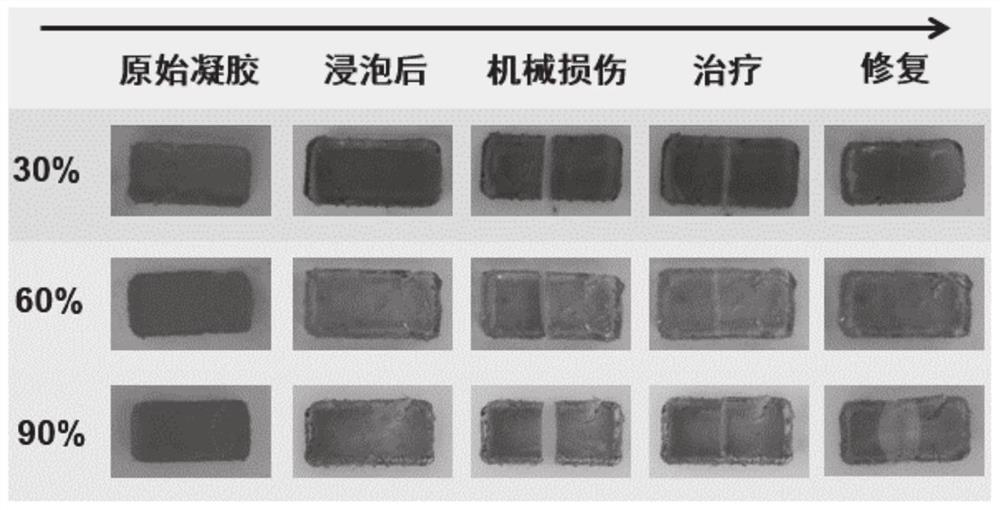
[0049] image 3 The ethylene glycol addition amount prepared for this embodiment is the repair diagram of the gel material after damage, when the gel material is mechanically damaged, smear 0.8M citric acid on its cross-section / sodium citrate buffer for repair. Here we introduce 0.1mg / mL acid-base indicator bromothymol blue (BTB) into the gel system, w...
Embodiment 2
[0054] The low-pH pristine hydrogel containing imine bonds obtained by cross-linking glutaraldehyde and BSA at pH 4 was immersed in NaHCO 3 The final gel material was obtained in aqueous ethylene glycol solution. Among them, the concentration of glutaraldehyde is 0.5wt%, the concentration of BSA is 12wt%, NaHCO 3 The concentration is 0.03M, and the mass fraction of ethylene glycol is 60%. After the gel was mechanically damaged, smearing 0.8M citric acid / sodium citrate buffer on its fracture surface could realize the repair of the structure and function of the gel, and the repair rate was finally calculated to be over 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com