Flavonoid tankyrase-2 inhibitor and preparation method and application thereof
A technology of tankyrase and flavonoids, applied in the field of flavonoid tankyrase 2 inhibitors and its preparation, can solve the problems of limiting tumor cell proliferation and growth, achieve good inhibitory activity, novel structure, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The preparation method of the flavonoid tankyrase 2 inhibitor uses 4-oxo-4H-1-benzopyran-2-carboxylic acid and aniline derivatives or benzohydrazide derivatives as starting materials , using uronium salt as a condensing agent, carrying out a condensation reaction in an organic solvent under the condition of adding a base, to obtain the flavonoid tankyrase 2 inhibitor;
[0049] The aniline derivatives are
[0050] Described benzoyl hydrazide derivative is
[0051] in:
[0052] R 1 selected from H, CH 3 , OCH 3 , Cl, Br or 3,4,5-tri-OCH 3 ;
[0053] R 2 selected from H, CH 3 , OCH 3 , Cl or Br.
[0054] Preferably the uronium salt is selected from HATU, HBTU, HCTU, TBTU, TSTU or TNTU. Preferably, the molar ratio of 4-oxo-4H-1-chromene-2-carboxylic acid to the condensing agent is 1:1˜1:5. Preferably, the molar ratio of 4-oxo-4H-1-benzopyran-2-carboxylic acid to aniline derivatives or benzohydrazide derivatives is 1:1˜1:3. Preferably, the molar ratio of 4-o...
Embodiment 1
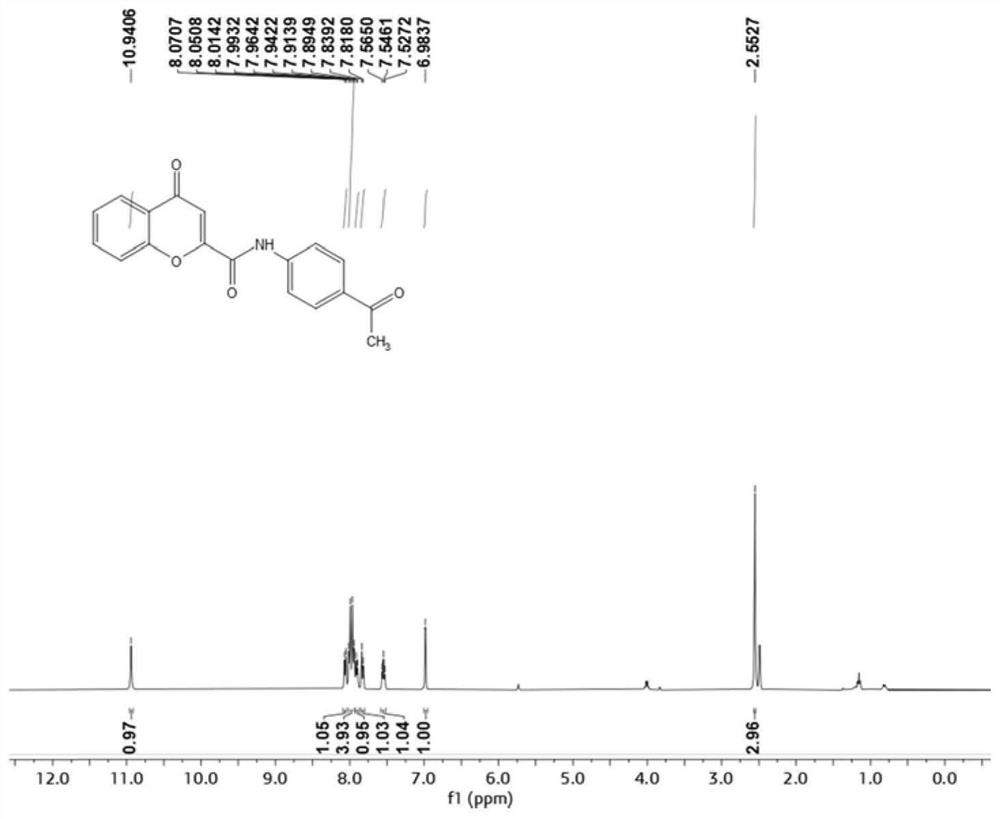
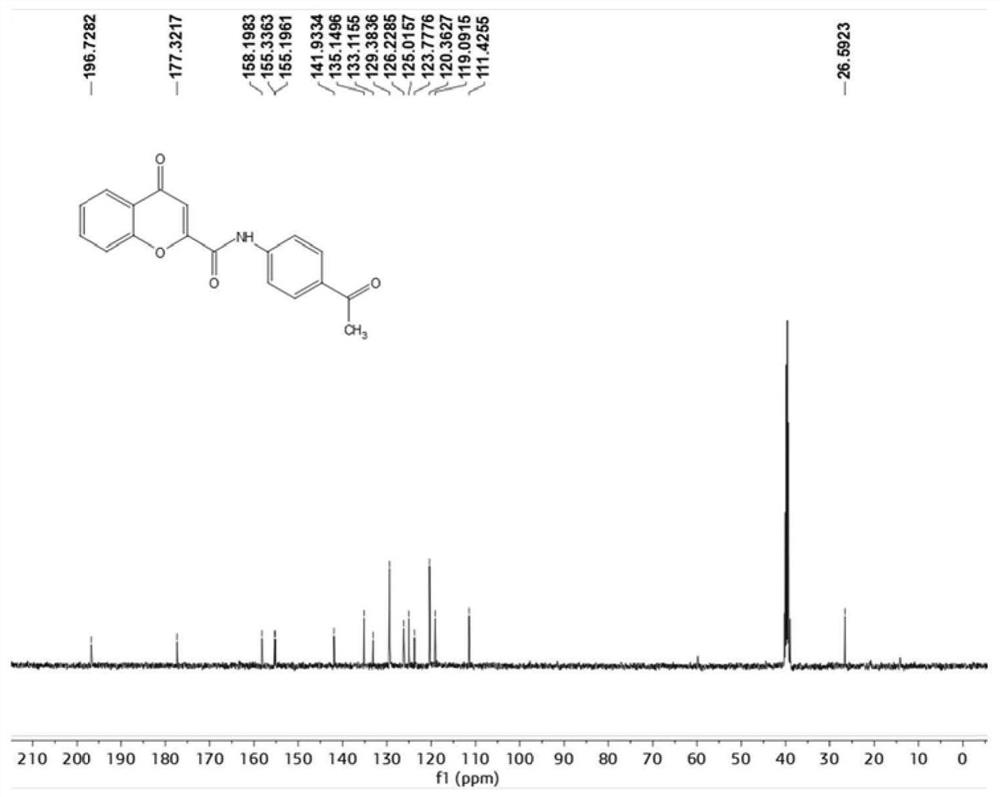
[0059] N-(4-Acetylphenyl)-4-oxo-4H-chromene-2-carboxamide
[0060]
[0061] 4-Oxo-4H-1-chromene-2-carboxylic acid (95mg, 0.5mmol) was dissolved in 25ml DMF, under ice-cooling conditions, HATU (228mg, 0.6mmol) and DIPEA ( 129mg, 1mmol) into a DMF solution containing 4-oxo-4H-1-chromene-2-carboxylic acid, slowly warming up to room temperature, and adding 4-aminoacetophenone (81mg, 0.6mmol) after 15 minutes . Stirring was continued at room temperature for 6 h until the reaction of 4-oxo-4H-1-chromene-2-carboxylic acid was complete. After the reaction was completed, the reaction solution was poured into ice water. Precipitate solid, collect solid and water, 10%Na 2 CO 3 , saline and ethanol sonication. The solid was purified by flash silica gel chromatography (petroleum ether / ethyl acetate=6:1) to obtain the product.
[0062] IC 50 The value is 8.74uM.
[0063] 1 H NMR (400MHz, DMSO)δ=10.94(s,1H),8.06(d,J=8.0Hz,1H),7.98(q,J=8.6Hz,4H),7.90(d,J=7.6Hz,1H ), 7.83(d, J=8.5...
Embodiment 2
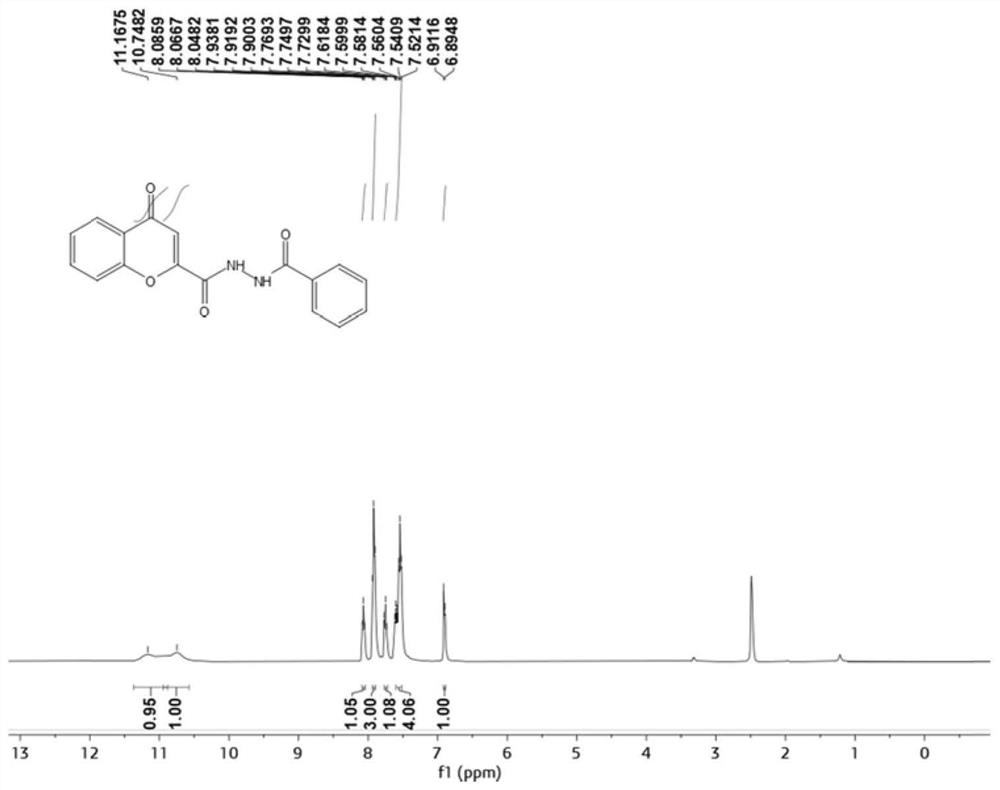
[0066] N'-Benzoyl-4-oxo-4H-benzopyran-2-carbohydrazide
[0067]
[0068] 4-Oxo-4H-1-chromene-2-carboxylic acid (95mg, 0.5mmol) was dissolved in 25ml DMF, under ice-cooling conditions, HATU (228mg, 0.6mmol) and DIPEA ( 129 mg, 1 mmol) into a DMF solution containing 4-oxo-4H-1-chromene-2-carboxylic acid, slowly warming up to room temperature, and adding benzohydrazide (82 mg, 0.6 mmol) after 15 minutes. Stirring was continued at room temperature for 6 h until the reaction of 4-oxo-4H-1-chromene-2-carboxylic acid was complete. After the reaction was completed, the reaction solution was poured into ice water. Precipitate solid, collect solid and water, 10%Na 2 CO 3 , saline and ethanol sonication. The solid was purified by flash silica gel chromatography (petroleum ether / ethyl acetate=4:1) to obtain the product.
[0069] IC 50 The value is 3.54uM.
[0070] 1 H NMR (400MHz, DMSO) δ11.17(s, 1H), 10.75(s, 1H), 8.07(t, J=7.5Hz, 1H), 7.92(t, J=7.6Hz, 3H), 7.76(d ,J=7.9Hz,1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com