Preparation method of carboprost
A carboprost and solvent technology, which is applied in the field of carboprost preparation, can solve the problems of complex post-processing and low combined yield, and achieve the effects of simple post-processing, low production cost and high combined yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
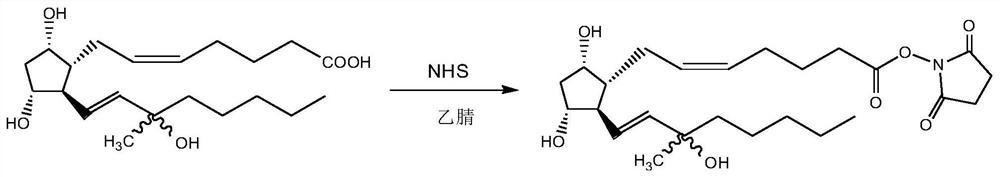
[0028] 1) Crude carboprost combined with NHS:
[0029]
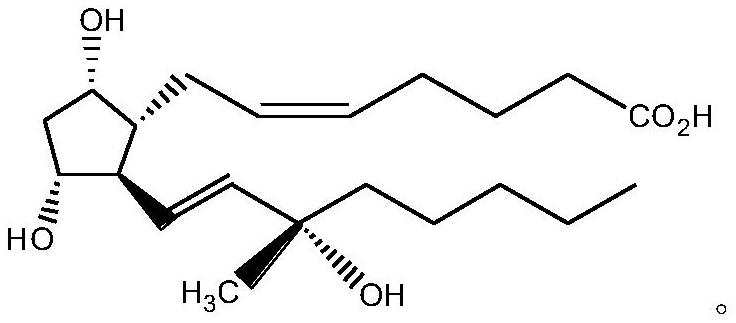
[0030] Add 150mL acetonitrile in the four-neck flask, add 10.00g of carboprost crude product (its carboprost content is 52.71% as detected by HPLC) in 150mL acetonitrile, then add 4.39g N, N'-carbonyldiimidazole, N 2 After replacement, let stand at room temperature for 2 hours.
[0031] Add 4.68g of N-hydroxysuccinimide dropwise into a four-necked flask, and keep it at room temperature for 2 hours after dropping. HPLC tracking shows that the reaction solution is concentrated under reduced pressure after the raw materials are completely reacted to obtain 16.99g of a light yellow amorphous substance; 1 H NMR (500 MHz, Chloroform-d): δ5.71(t,1H),5.69(m,1H),5.46-5.44(m,2H),3.29(t,1H),3.21(m,1H), 2.76-2.73(m,4H),2.26-2.23(m,3H),2.15-2.11(t,3H),1.98-1.96(t,2H),1.92-1.90(t,2H),1.81(t,2H ), 1.65(d,1H), 1.60(m,2H), 1.44-1.41(m,5H), 1.33-1.29(m,6H), 0.96(t,3H).
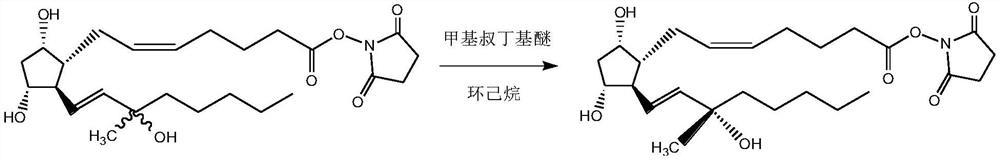
[0032] 2) Preparation of carboprost ester:
[0033]
[003...
Embodiment 2
[0039] 1) Crude carboprost combined with NHS:
[0040]
[0041] Add 150mLDMF in the four-neck flask, add 10.00g of carboprost crude product (HPLC detects that its carboprost content is 51.54%) in 150mLDMF, then add 4.39g N,N'-carbonyldiimidazole, N 2 After replacement, let stand at room temperature for 2 hours.
[0042] 4.68g of N-hydroxysuccinimide was added dropwise into a four-neck flask, and kept at room temperature for 2 hours after the dropping was completed. HPLC tracking showed that the reaction solution was concentrated under reduced pressure to obtain 16.78g of a light yellow amorphous substance after the completion of the reaction of the raw materials.
[0043] 2) Preparation of carboprost ester:
[0044]
[0045] Add all the light yellow amorphous substance obtained in the previous step into the four-neck flask, then add 100mL methyl tert-butyl ether and 10mL cyclohexane, heat up to 40°C while stirring, keep warm for 2h, then cool down to room temperature, a...
Embodiment 3
[0050] 1) Crude carboprost combined with NHS:
[0051]
[0052] Add 150mL tetrahydrofuran in the four-necked flask, add 10.00g of carboprost crude product (its carboprost content is 53.55% as detected by HPLC) in 150mL tetrahydrofuran, then add 4.39g N,N'-carbonyldiimidazole, N 2After replacement, let stand at room temperature for 2 hours.
[0053] Add 4.68g of N-hydroxysuccinimide dropwise into a four-necked flask, and keep it at room temperature for 2 hours after dropping. HPLC tracking shows that the reaction solution is concentrated under reduced pressure to obtain 17.02g of a light yellow amorphous substance.
[0054] 2) Preparation of carboprost ester:
[0055]
[0056] Add all the light yellow amorphous substance obtained in the previous step into the four-neck flask, then add 100mL of methyl tert-butyl ether and 10mL of methylcyclohexane, heat up to 30°C while stirring, keep warm for 2h, then cool down to room temperature, and statically Set aside for 2h, a pal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com