D-pi-A type tetraphenylethynyl phenyl substituted pyridine conjugated luminescent small molecule and synthesis method thereof
A technology of ethynylphenyl and tetraphenylethylene, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., to achieve good photophysical properties, good thermal stability, and excellent fluorescent properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
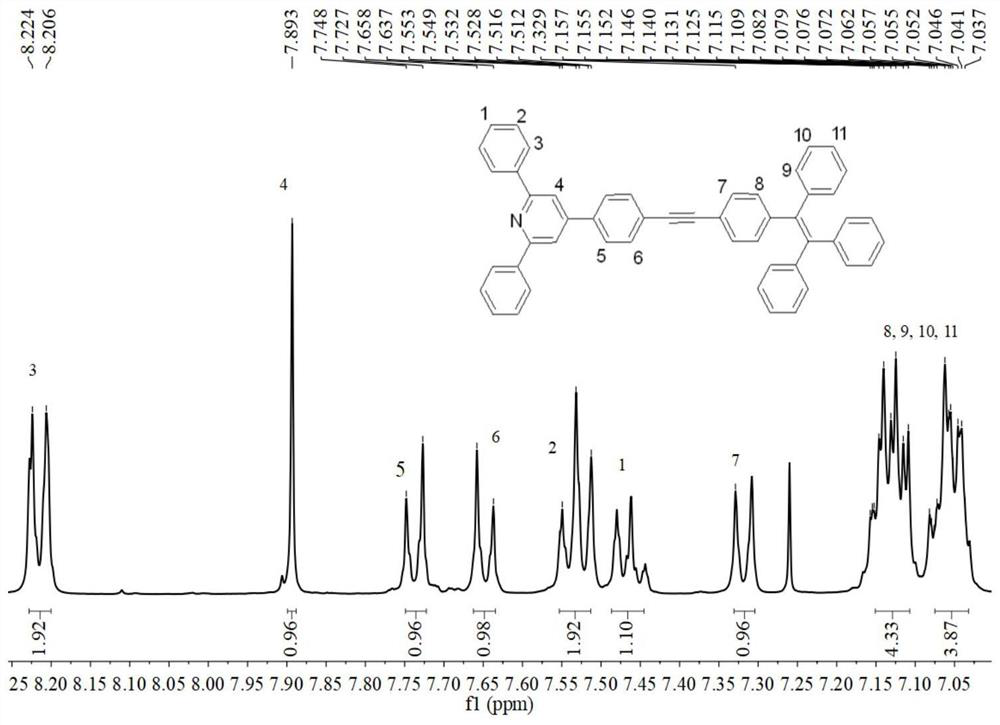
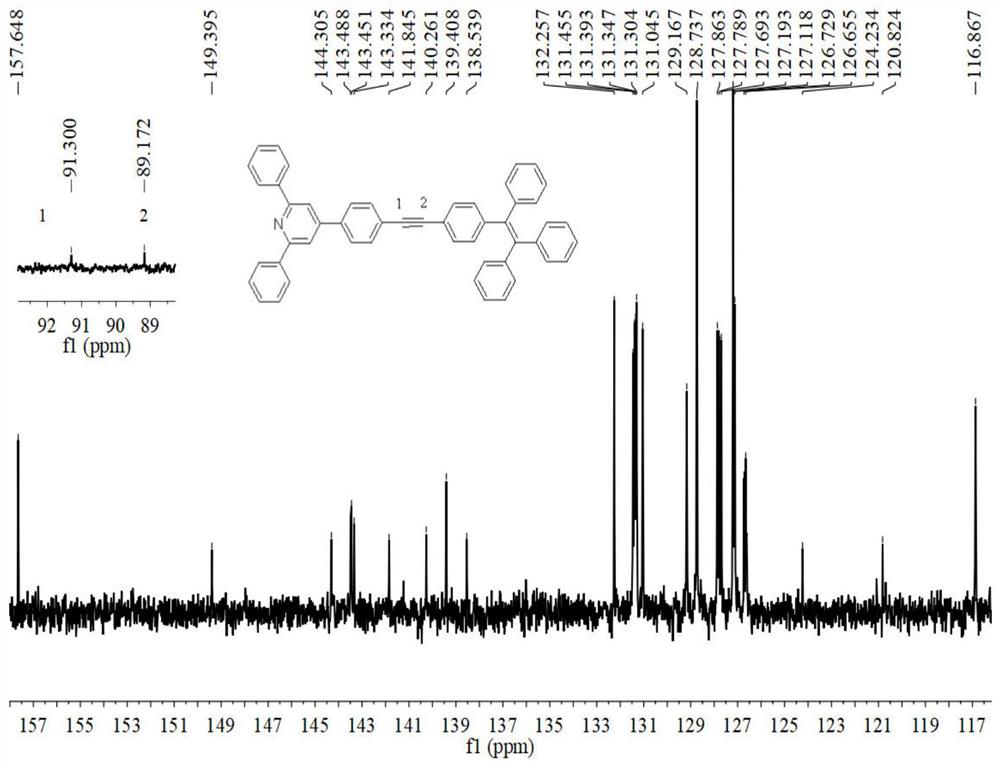
Examples
Embodiment 1
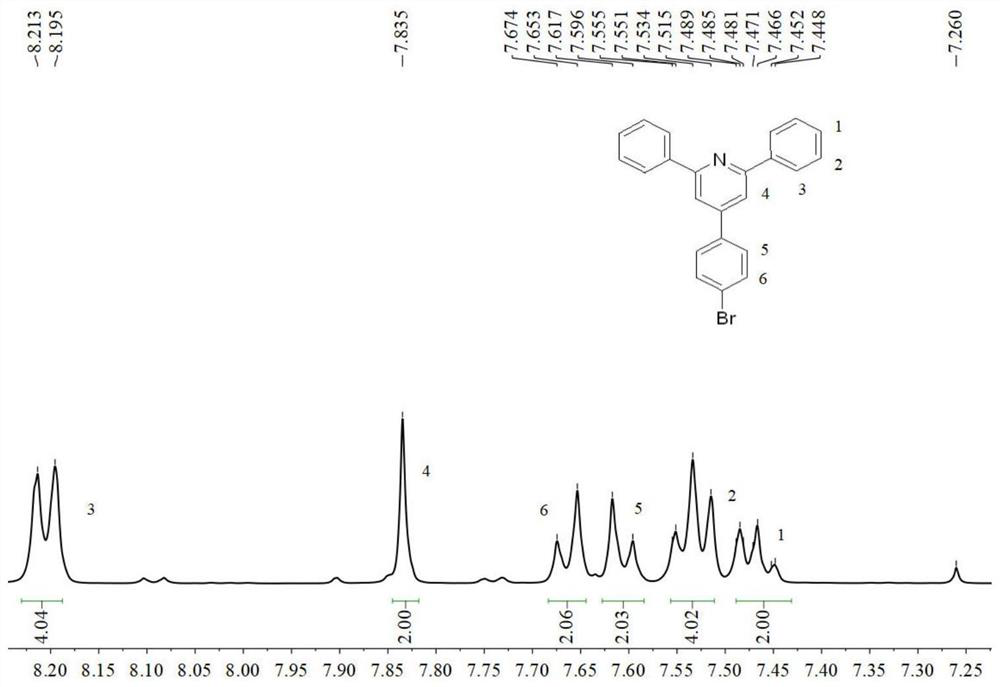
[0058] The preparation of 2,6-diaryl-4-(4-bromophenyl)pyridine (4) (synthetic methods are the same, but the raw materials are different): add 4-bromobenzaldehyde (9.95 g, 50mmol), arylacetophenone (105mmol), ammonium acetate (27.00g, 350mmol) and glacial acetic acid (40mL). Under the protection of nitrogen, react at a temperature of 110-130° C. for 7 h, and track the reaction by thin layer chromatography (TLC). After the reaction was complete, the reaction was stopped and cooled to room temperature, and a brownish-yellow viscous substance was precipitated. Suction filtration and washing with absolute ethanol for 2-3 times, the crude product was dried and recrystallized with absolute ethanol to obtain the target compound 4.
[0059] 2,6-Diphenyl-4-(4-bromophenyl)pyridine (4a): Yield: 86%; white needle-like solid, mp=131~132℃. 1 H NMR (400MHz, CDCl 3 ):δ(ppm)=8.20(d,J=8.0Hz,4H),7.83(s,2H),7.66(d,J=8.0Hz,2H),7.61(d,J=8.0Hz,2H), 7.55-7.51(m,4H),7.49-7.45(m,4H),3.88(s,6H).
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com