A kind of photoresponsive maleopimaric acid-based azobenzene amphiphilic polymer, preparation method and application thereof
A technology of pimaric acid-based azobenzene and amphiphilic polymer, which is applied in the field of maleopimaric acid-based azobenzene amphiphilic polymer, can solve the problem of low profit in rosin industry, insufficient international competitiveness, low deep processing rate, etc. problem, to achieve the effect of excellent photoresponse performance, good aggregation ability, good reversibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
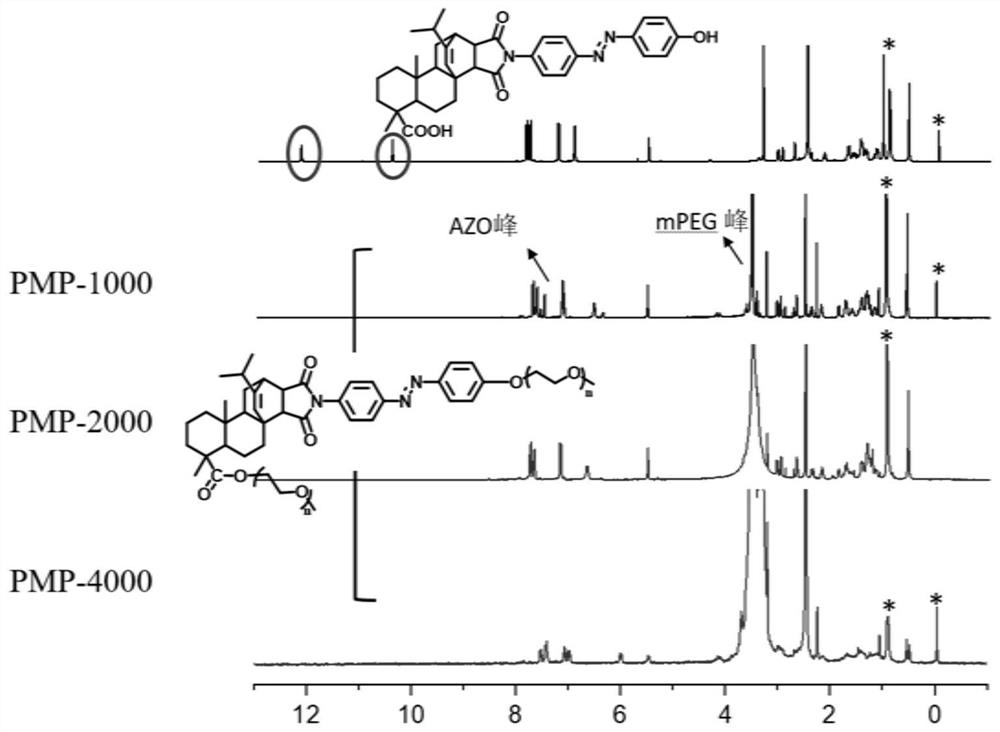
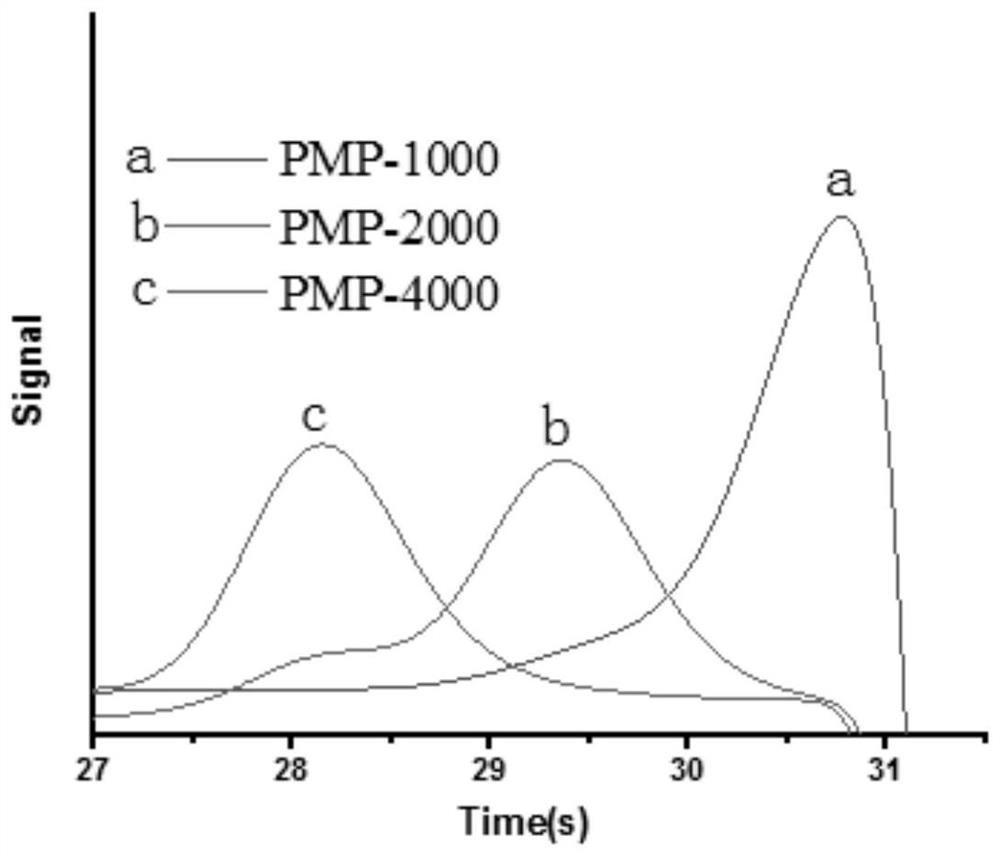
[0040] Polyethylene glycol monomethyl ether (Mn=1000) (4g, 0.004mol) and triethylamine (0.2g, 0.02mol) were placed in a 100mL round bottom flask, dissolved in 30mL of dry dichloromethane, the reaction mixture was Cool to 0°C; then add 4-toluenesulfonyl chloride (3.80g, 0.02mol), react at 0°C for 2h, then heat up to 25°C, react for 12h, the reaction is terminated, precipitate with ether and recrystallize with ethanol to obtain p-toluene Sulfopolyethylene glycol monomethyl ether.
[0041] p-Toluenesulfopolyethylene glycol monomethyl ether (1.5 g, 1.3 mmol) and MPA-AZO-OH (0.77 g, 1.3 mmol) were placed in a round-bottomed flask, respectively, with 50 mL of DMF and K 2 CO 3 (0.2 g, 1.3 mmol) dissolved. The reaction mixture was stirred in a preheated oil bath at 50°C for 24 hours, then the reaction was stopped. The product was precipitated in excess cold ether, recrystallized in ethanol, dried for 48 hours, dialyzed in water for 3 days, and freeze-dried to obtain the maleopimari...
Embodiment 2
[0045] Put polyethylene glycol monomethyl ether (Mn=2000) (4g, 0.002mol) and triethylamine (0.1g, 0.01mol) into a 100mL round bottom flask, dissolve in 30mL of dry dichloromethane, the reaction mixture Cool to 0°C; then add 4-toluenesulfonyl chloride (1.90g, 0.01mol), react at 0°C for 2h, then heat up to 25°C, react for 12h, the reaction is terminated, precipitate with ether and recrystallize with ethanol to obtain p-toluene Sulfopolyethylene glycol monomethyl ether.
[0046] p-Toluenesulfopolyethylene glycol monomethyl ether (1.5 g, 0.7 mmol) and MPA-AZO-OH (0.42 g, 0.7 mmol) were placed in a round-bottomed flask, respectively, with 50 mL of DMF and K 2 CO 3 (0.1 g, 0.7 mmol) dissolved. The reaction mixture was stirred in a preheated oil bath at 50°C for 24 hours, then the reaction was stopped. The product was precipitated in excess cold ether and recrystallized in ethanol, dried for 48 hours, dialyzed in water for 3 days, and freeze-dried to obtain the maleopimaric acid-b...
Embodiment 3
[0050] Polyethylene glycol monomethyl ether (Mn=4000) (4g, 0.001mol) and triethylamine (0.05g, 0.005mol) were put into a 250mL round bottom flask, dissolved in 100mL of dry dichloromethane, the reaction mixture was Cool to 0°C; then add 4-toluenesulfonyl chloride (0.90g, 0.005mol), react at 0°C for 2h, then heat up to 25°C, react for 12h, the reaction is terminated, precipitate with ether and recrystallize with ethanol to obtain Tosyl sulfopolyethylene glycol monomethyl ether.
[0051] p-Toluenesulfopolyethylene glycol monomethyl ether (1.5 g, 0.375 mmol) and MPA-AZO-OH (0.11 g, 0.19 mmol) were placed in a round-bottomed flask, respectively, with 50 mL of DMF and K 2 CO 3 (0.05 g, 0.375 mmol) dissolved. The reaction mixture was stirred in a preheated oil bath at 50°C for 24 hours, then the reaction was stopped. The product was precipitated in excess cold ether, recrystallized in ethanol, dried for 48 hours, dialyzed in water for 3 days, and freeze-dried to obtain the maleop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com