Sorafenib-ruthenium complex as well as preparation method and application thereof
A ruthenium complex, sorafenib technology, applied in the field of organic targeted drugs and synthesis, can solve the problems of unsatisfactory drug efficacy, unsatisfactory active drug release effect, etc., and achieve high efficiency, low toxicity, drug resistance, good cell light The effect of less toxicity and by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
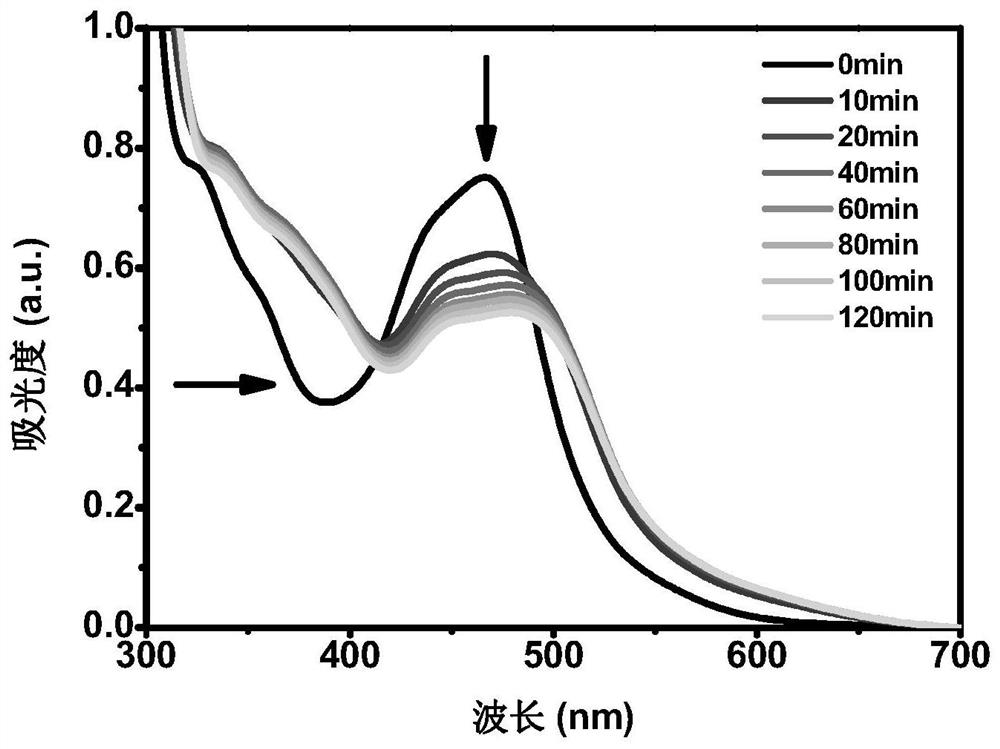
Image
Examples
preparation example Construction
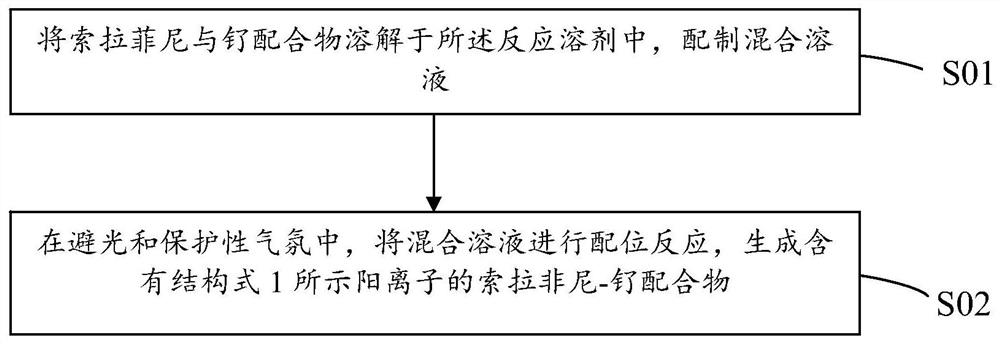
[0030] Correspondingly, the embodiment of the present invention also provides a preparation method of the above-mentioned sorafenib-ruthenium complex. The process flow of the preparation method of the embodiment of the present invention Sorafenib-ruthenium complex is shown in the figure, the above structural formula 1 0 The preparation method of shown Sorafenib-ruthenium complex comprises the steps:
[0031] S01: dissolving Sorafenib and the ruthenium complex in the reaction solvent to prepare a mixed solution;
[0032] S02: In a dark and protective atmosphere, the mixed solution is subjected to a coordination reaction to generate a Sorafenib-ruthenium complex containing a cation shown in the following structural formula 1:
[0033] Wherein, the reactant ruthenium complex in step S01 can be a ruthenium complex commonly used in the art, such as the following chemical structural formula is the following structural formula 1 A Shown ruthenium complex; The chemical structural fo...
Embodiment 1
[0051] This embodiment provides a Sorafenib-ruthenium complex and its preparation method. The structural formula of the Sorafenib-ruthenium complex in this embodiment is the structural formula 1 in the following chemical reaction formula 1 1 shown.
[0052] The preparation method of the present embodiment Sorafenib-ruthenium complex comprises the following steps:
[0053] S1: Add ruthenium precursor ([Ru(bpy) 2 (Cl) 2 ]) (96.87mg, 0.20mmol) and Sorafenib (Sorafenib) (111.56mg, 0.24mmol) were added in a 1:4 volume ratio of water: ethanol mixed solvent (10mL);
[0054] S2: Purify by column chromatography after reflux reaction at 80°C for 6 hours in the dark to obtain the structural formula 1 1 The sorafenib-ruthenium complex shown.
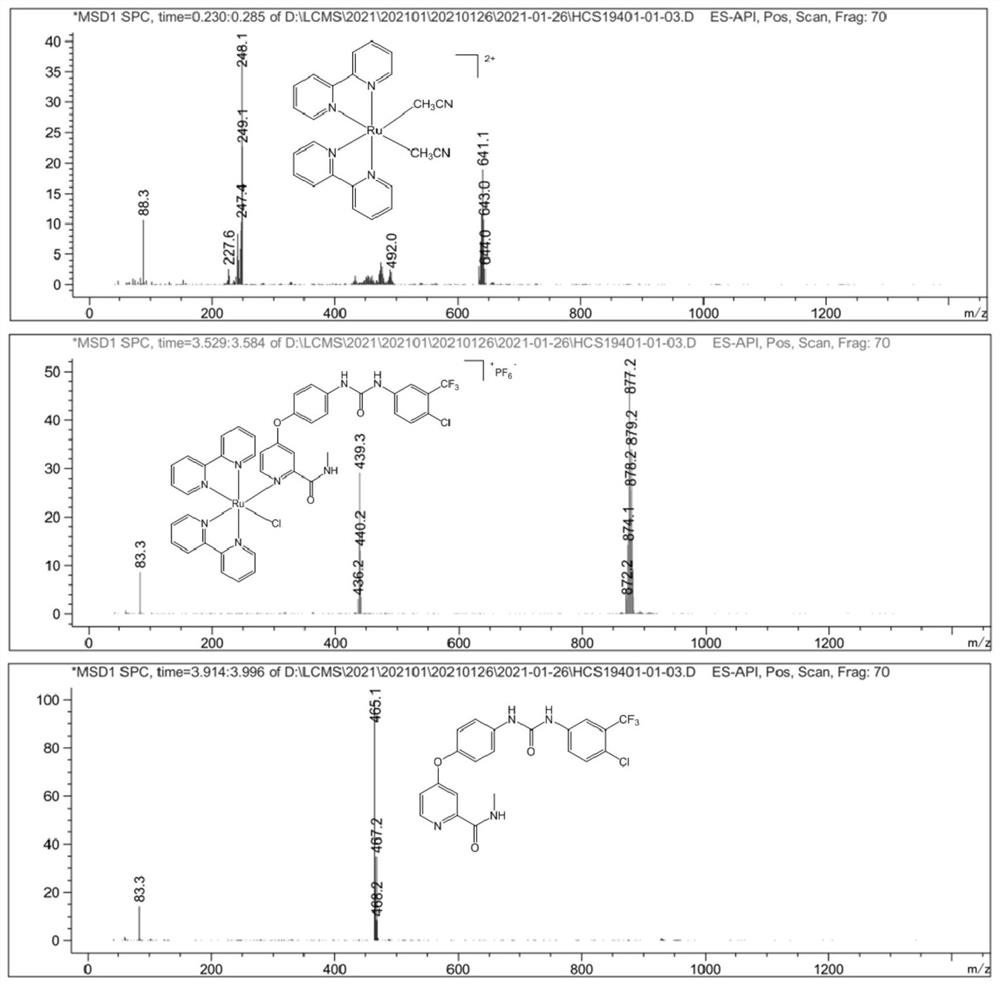
[0055] After purification, structural formula 1 1 The yield of the sorafenib-ruthenium complex shown was 76%. Characterized by mass spectrometry and NMR, the ruthenium complex was successfully prepared. The molecular formula is: C 41 h 32 C...
Embodiment 2
[0059] This embodiment provides a Sorafenib-ruthenium complex and its preparation method. The structural formula of the Sorafenib-ruthenium complex in this embodiment is the structural formula 1 in the following chemical reaction formula 2 2 shown.
[0060] The preparation method of the present embodiment Sorafenib-ruthenium complex comprises the following steps:
[0061] The mixed solution after the reflux reaction in step S2 of Example 1 was cooled to room temperature and filtered to remove unreacted substances, the filtrate was collected, and a 10-fold excess saturated ammonium hexafluorophosphate aqueous solution (NH 4 PF 6 ), a dark red precipitate was precipitated, and the liquid was placed at a low temperature to precipitate more precipitates, filtered and washed twice with ice-deionized water and ether, respectively, to obtain a dark red solid with a yield of 76%.
[0062] Characterized by mass spectrometry and NMR, the ruthenium complex was successfully prepared. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com