Multiple antibodies against human TSLP and uses thereof
A technology of monoclonal antibody and bispecific antibody, which is applied in the fields of antibodies, anti-inflammatory agents, allergic diseases, etc., and can solve problems such as the inability to control the condition of asthmatic patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0206] Example 1: Preparation of recombinant protein
[0207]The preparation of anti-TSLP antibodies requires the use of a variety of different recombinant proteins, including a long version of the human TSLP extracellular domain (hTSLP1-ECD, SEQ ID NO: 1), a short version of the human TSLP extracellular domain (hTSLP2-ECD) , SEQ ID NO:2), mouse TSLP extracellular domain (mTSLP-ECD, SEQ ID NO:3) and cynomolgus monkey TSLP extracellular domain (mfTSLP1-ECD, SEQ ID NO:4). These proteins have a large number of post-translational modifications (such as glycosylation or disulfide bonds, etc.), so the use of mammalian cell expression systems will be more conducive to maintaining the structure and function of recombinant proteins. In addition, in order to avoid the influence of the furin recognition sites in hTSLP1, hTSLP2 and mfTSLP1 on the activity of TSLP proteins, hTSLP1 mutants (hTSLP1-m, SEQ ID NO: 5) with the furin recognition sites deleted (hTSLP1-m, SEQ ID NO: 5), hTSLP2 Mu...
Embodiment 2
[0210] Example 2: Construction and screening of TSLP mouse immune library based on immobilized light chain
[0211] In order to construct a TSLP bispecific antibody based on a common light chain, the variable region of the light chain of the anti-TSLP monoclonal antibody with a specific epitope was selected to match the variable region of the mouse heavy chain that has undergone affinity maturation of the TSLP antigen in vivo, and conventional molecular biology methods were used. A single-chain antibody (scFv) library was constructed for screening anti-TSLP monoclonal antibodies against different epitopes.
[0212] After immunizing BALB / c mice aged 6-8 weeks with hTSLP1-m-His recombinant protein as immunogen, spleen cells were collected. Mouse spleen lymphocytes were isolated using mouse lymphocyte separation medium (Daktronics Biotechnology Co., Ltd., CAT#DKW33-R0100), and total cell RNA extraction kit (Tiangen Biochemical Technology (Beijing) Co., Ltd., CAT#DP430), total RN...
Embodiment 3
[0214] Example 3: Identification of anti-hTSLP murine monoclonal antibodies
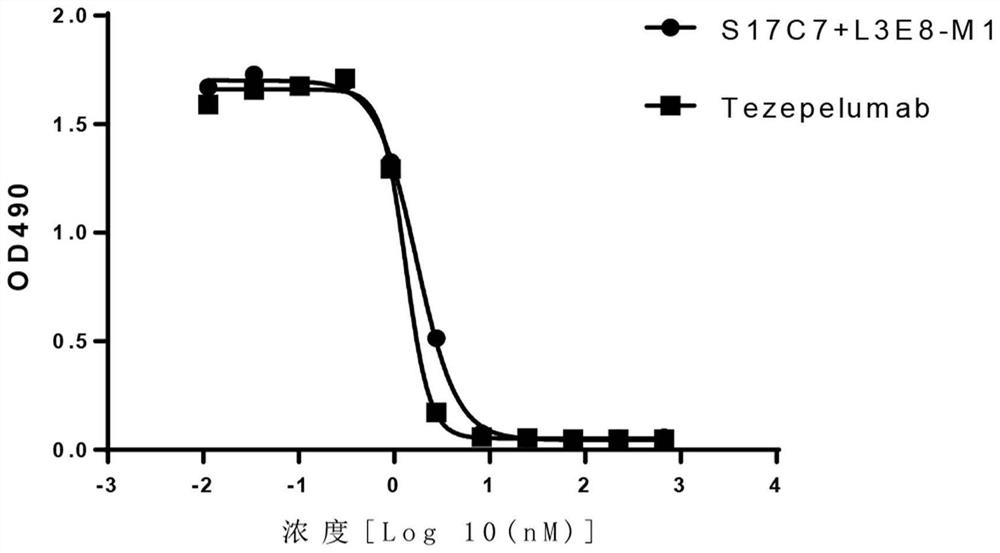
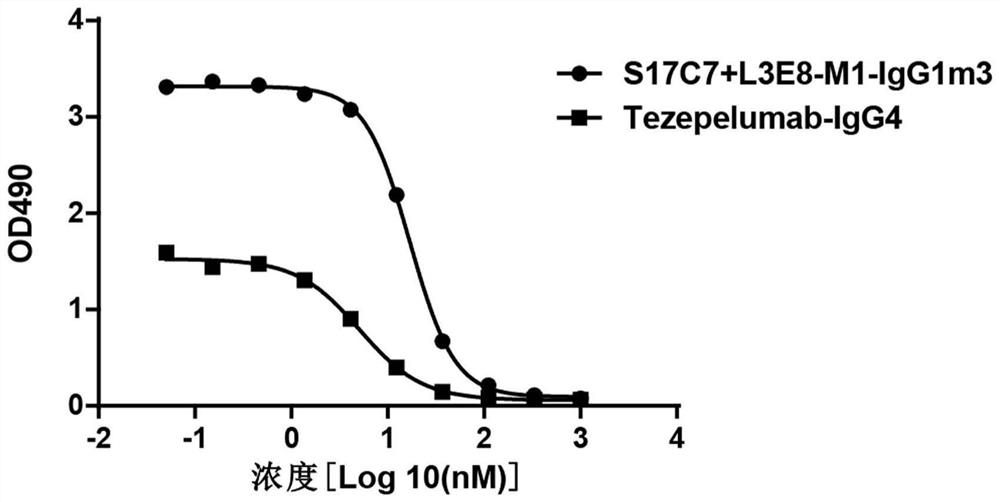
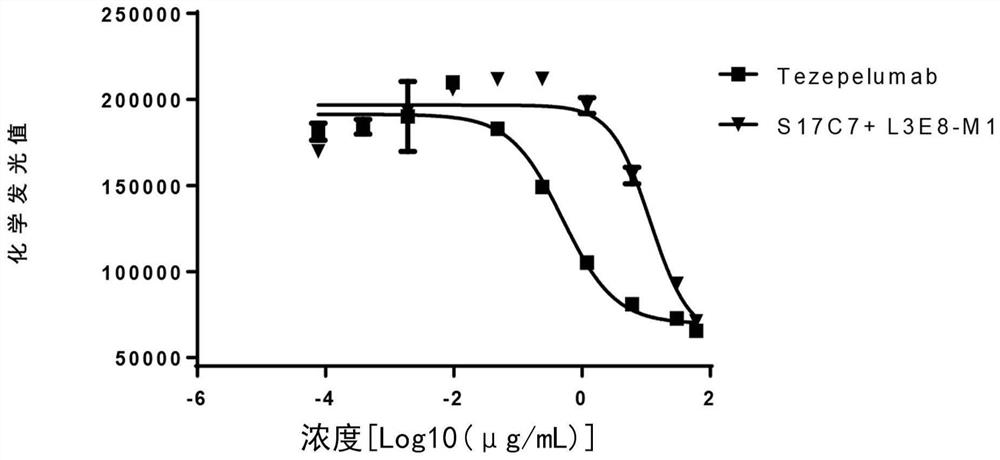
[0215] Using conventional molecular biology methods, nucleic acid molecules encoding the light variable region and heavy chain variable region of S17C7+L3E8-M1 were cloned into a eukaryotic expression vector to prepare and express a full antibody. At the same time, the humanized anti-TSLP monoclonal antibody Tezepelumab was prepared as a positive control with reference to US Patent US9284372B2 (the amino acid sequence of the variable region of the heavy chain is shown in SEQ ID NO: 22; the amino acid sequence of the variable region of the light chain is shown in SEQ ID NO: 23 ).
[0216] 3.1 Affinity analysis of recombinant anti-TSLP monoclonal antibody S17C7+L3E8-M1
[0217] The affinity of anti-TSLP antibodies was determined by surface plasmon resonance using a Biacore X100. Amino Coupling Kit (BR-1000-50), Human Antibody Capture Kit (BR-1008-39), CM5 Chip (BR100012) and 10×HBS-EP (BR100669) with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com