Sitagliptin impurity, and preparation method and detection method thereof
A kind of technology of sitagliptin and detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043] The present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments.
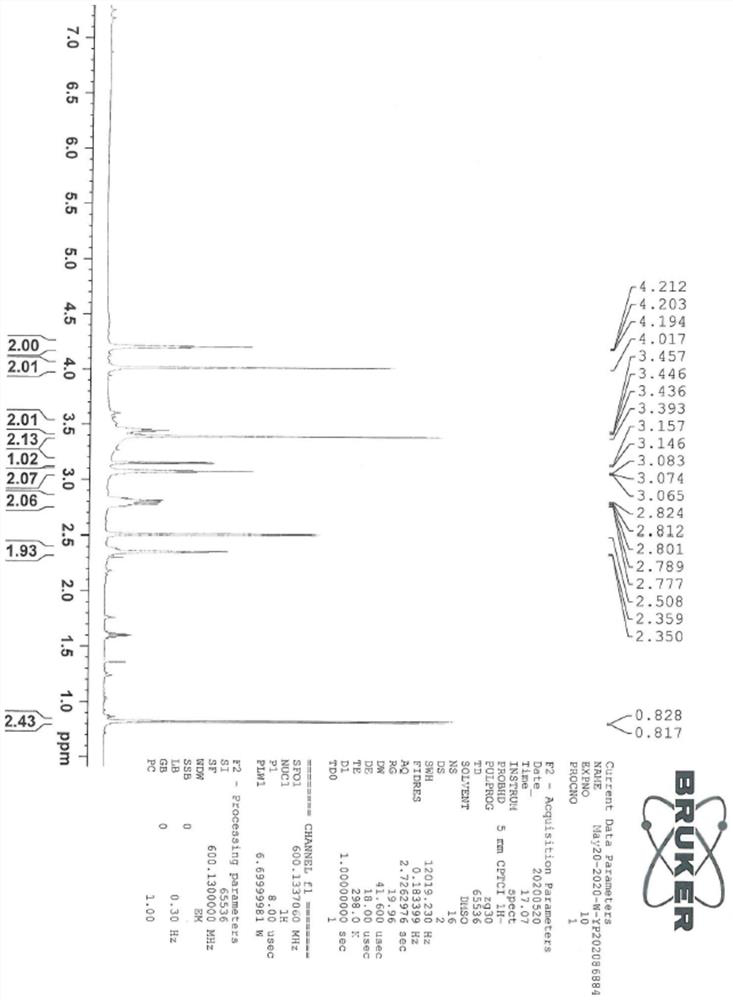
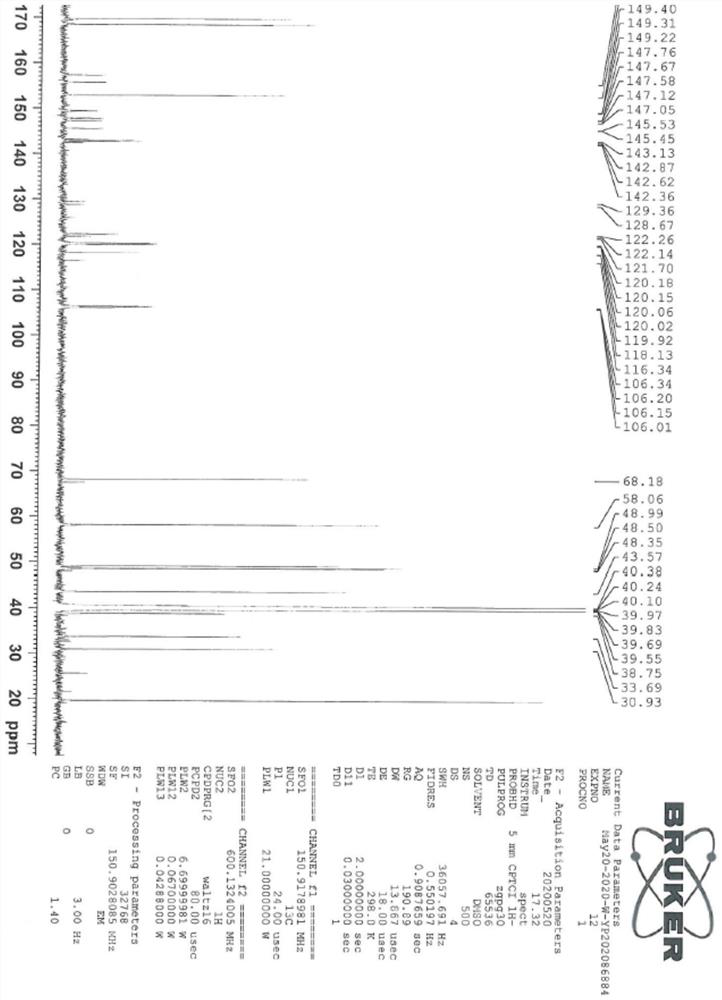
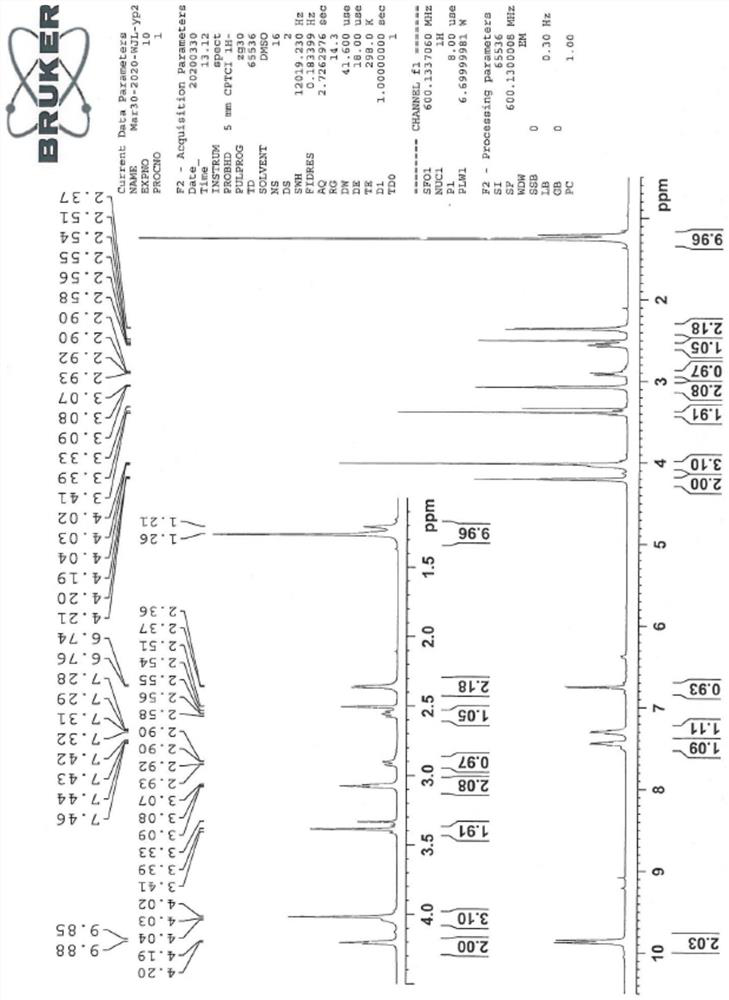
[0044] The sitagliptin impurity of the present invention is that the sitagliptin impurity is (R)-3-amino-N'-(2-(3-(trifluoromethyl)-5,6-dihydro-[1,2 ,4] Triazolo[4,3-a]pyrazin-7(8H)-yl)acetyl)-4-(2,4,5-trifluorophenyl)butanohydrazide, whose structural formula is:
[0045]
[0046] In the present embodiment, the preparation method of above-mentioned sitagliptin impurity is:
[0047] In the solution that 19g hydrazine hydrate is dissolved in 2000ml isopropanol, at 0-5 ℃, add di-tert-butyl dicarbonate solution (50g di-tert-butyl dicarbonate is dissolved in 250ml isopropanol), react The material was stirred at room temperature until the reaction was monitored by TLC. After the reaction was completed, the reaction material was distilled under vacuum to obtain the product of formula III, which was dissolved in 1000 ml of tetrahydrofuran and cool...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com