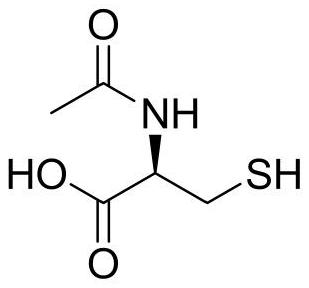
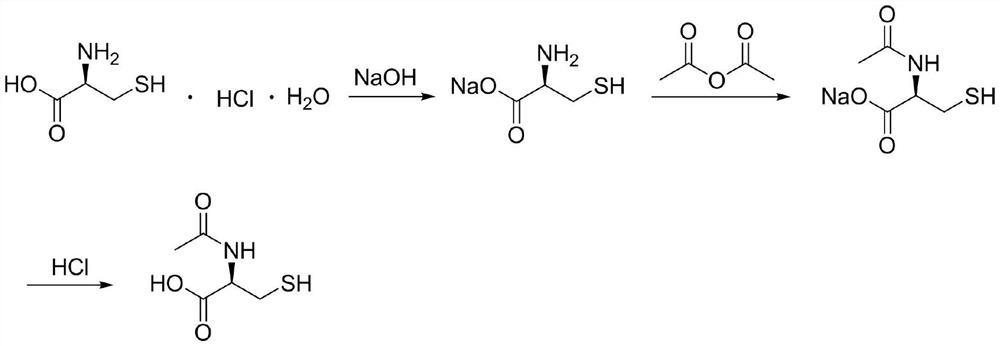
Synthesis method of N-acetyl-L-cysteine
A technology of cysteine and synthesis method, applied in the field of amino acid synthesis, which can solve the problems of large fluctuations in impurity content, difficult control of side reactions, high energy consumption, etc., achieve high molar yield, and avoid the effect of thermal decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Put 150kg of L-cysteine hydrochloride monohydrate into the reaction kettle, then pump in 300kg of pure water, protect it with nitrogen, stir and cool down to -5°C; add 256kg of 40% sodium hydroxide solution, drop it, and keep it warm for -5 Stir at ℃ for 30 minutes; add 90 kg of acetic anhydride dropwise, after dropping, keep warm at -5°C and stir for 30 minutes; rise to an internal temperature of 90°C and react for 4 hours, then concentrate under reduced pressure to dryness; add 150 kg of pure water, add 247 kg of 9N hydrochloric acid dropwise, and finish dropping Cool down to -5°C to crystallize for 3 hours; filter and dry to obtain 124.3 kg of crude N-acetyl-L-cysteine;
[0044] Put 124.3kg of crude N-acetyl-L-cysteine into the reaction kettle, then pump in 124kg of pure water, protect it with nitrogen, heat up to 90°C to dissolve, then drop to -5°C for crystallization for 3 hours; filter and dry to obtain N-acetyl-L-cysteine refined product 111.9kg.
Embodiment 2
[0046] Put 600kg of L-cysteine hydrochloride monohydrate into the reaction kettle, then pump in 1200kg of pure water, protect it with nitrogen, stir and cool down to 5°C; add 2732kg of 15% sodium hydroxide solution, drop it, keep it warm at 5°C and stir 5min; add 340kg of acetic anhydride dropwise, keep warm at 5°C and stir for 5min; rise to an internal temperature of 70°C for 1 hour, then concentrate to dryness under reduced pressure; add 600kg of pure water, dropwise add 1460kg of 6N hydrochloric acid, drop the temperature to 5 ℃ for 1 hour; filter and dry to obtain 499.8kg of crude N-acetyl-L-cysteine;
[0047] Put 499.8kg of crude N-acetyl-L-cysteine into the reaction kettle, then pump in 500kg of pure water, protect it with nitrogen, heat up to 70°C to dissolve, then drop to 5°C for crystallization for 1 hour; filter and dry to obtain N - Acetyl-L-cysteine refined product 449.9kg.
Embodiment 3
[0049] Put 500kg of L-cysteine hydrochloride monohydrate into the reaction kettle, then pump in 1000kg of pure water, protect it with nitrogen, stir and cool down to 10°C; add 1140kg of 30% sodium hydroxide solution, drop it, keep it warm at 10°C and stir 20min; add 300kg of acetic anhydride dropwise, after dropping, keep warm at 10°C and stir for 20min; rise to an internal temperature of 80°C for 3 hours, then concentrate to dryness under reduced pressure; add 500kg of pure water, dropwise add 2446kg of 3N hydrochloric acid, drop the temperature to 10°C ℃ crystallization for 2 hours; filter and dry to obtain 418.6 kg of crude N-acetyl-L-cysteine;
[0050] Put 418.6kg of crude N-acetyl-L-cysteine into the reaction kettle, then pump in 420kg of pure water, protect it with nitrogen, heat up to 50°C to dissolve, then drop to 10°C for crystallization for 2 hours; filter and dry to obtain N - Acetyl-L-cysteine refined product 376.7kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com