Modified carbon nitride loaded noble metal-based electrocatalyst as well as preparation method and application thereof
An electrocatalyst, carbon nitride technology, applied in circuits, electrical components, battery electrodes, etc., can solve the problems of poor thermodynamic stability of carbon-based materials, aggravating the agglomeration of precious metal nanoparticles, destroying the structural integrity of catalysts, etc. Enhanced structural stability and long-term cycle stability, and the effect of simple and controllable preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] In one embodiment of the present disclosure, a method for preparing a modified carbon nitride-supported noble metal-based electrocatalyst includes:
[0035] (1), placing carbon nitride in hydrochloric acid for surface protonation;
[0036] (2), mixing the protonated carbon nitride and the heteroatom source for heat treatment to obtain modified carbon nitride;
[0037] (3) After mixing the modified carbon nitride and the noble metal source, the noble metal nanoparticles are reacted and supported.
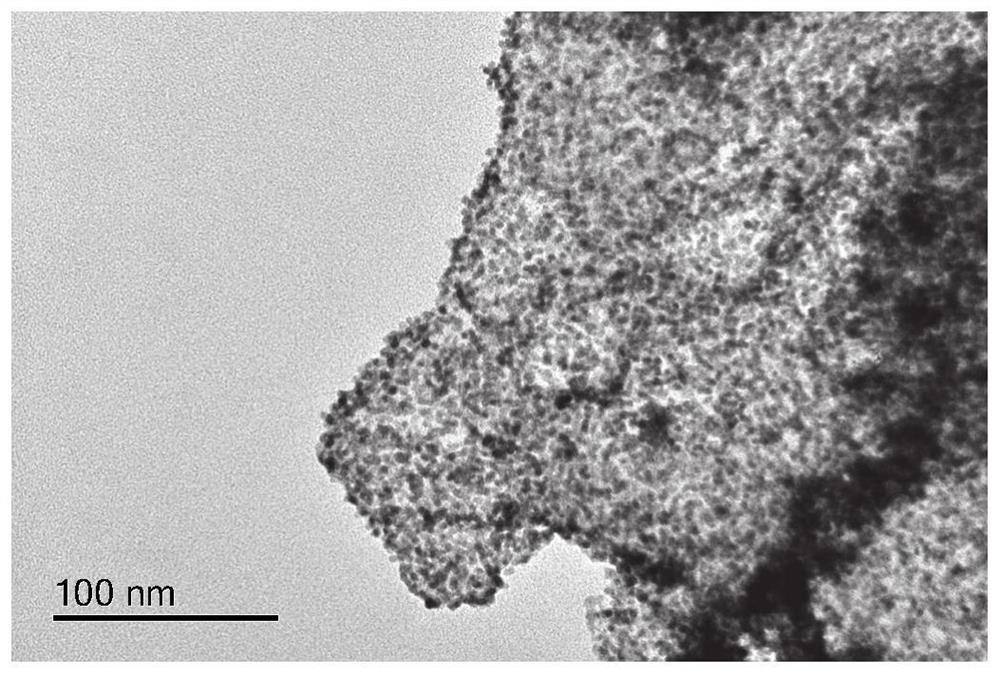
[0038]The preparation method provided by the disclosure is simple, and the obtained electrocatalyst is uniformly dispersed without agglomeration. The preparation method can reduce the loaded amount of the noble metal nanoparticles, and effectively control the size of the noble metal nanoparticles at 3-10nm, which is conducive to maximizing the catalytic activity of the noble metals.
[0039] Further, in step (1), the preparation method of carbon nitride comprises heat-treati...
Embodiment 1
[0063] A modified carbon nitride carrier-supported platinum oxygen reduction reaction electrocatalyst, the preparation method specifically comprising:
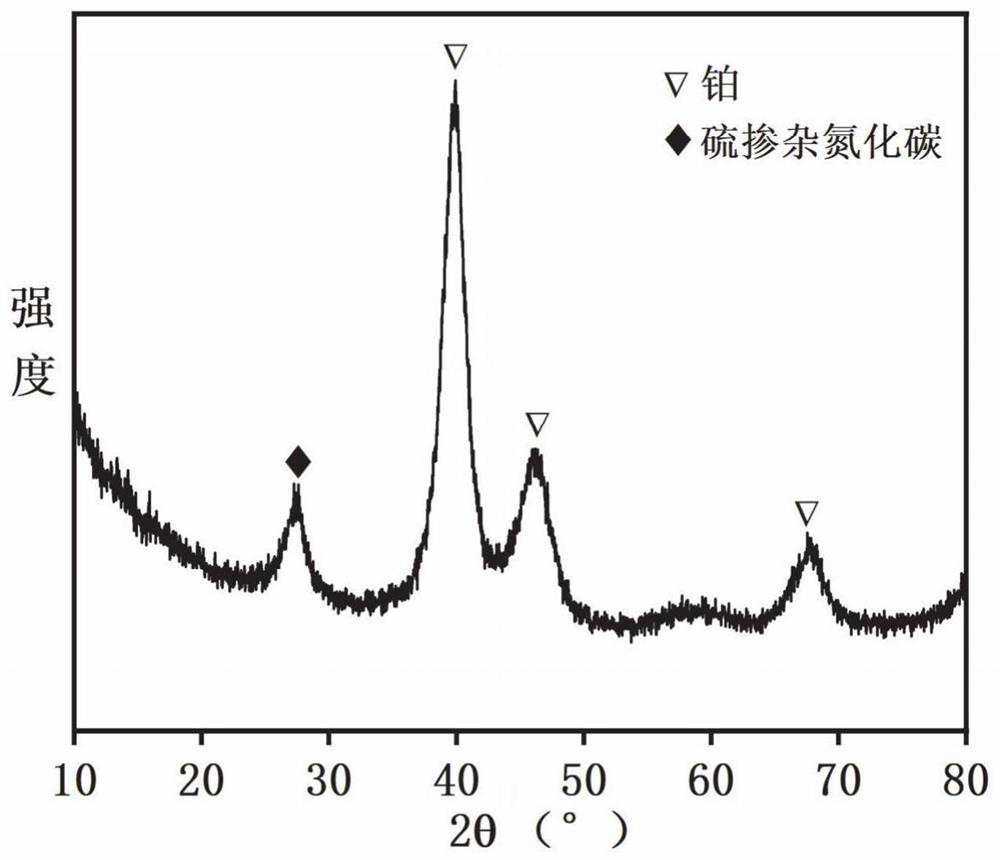
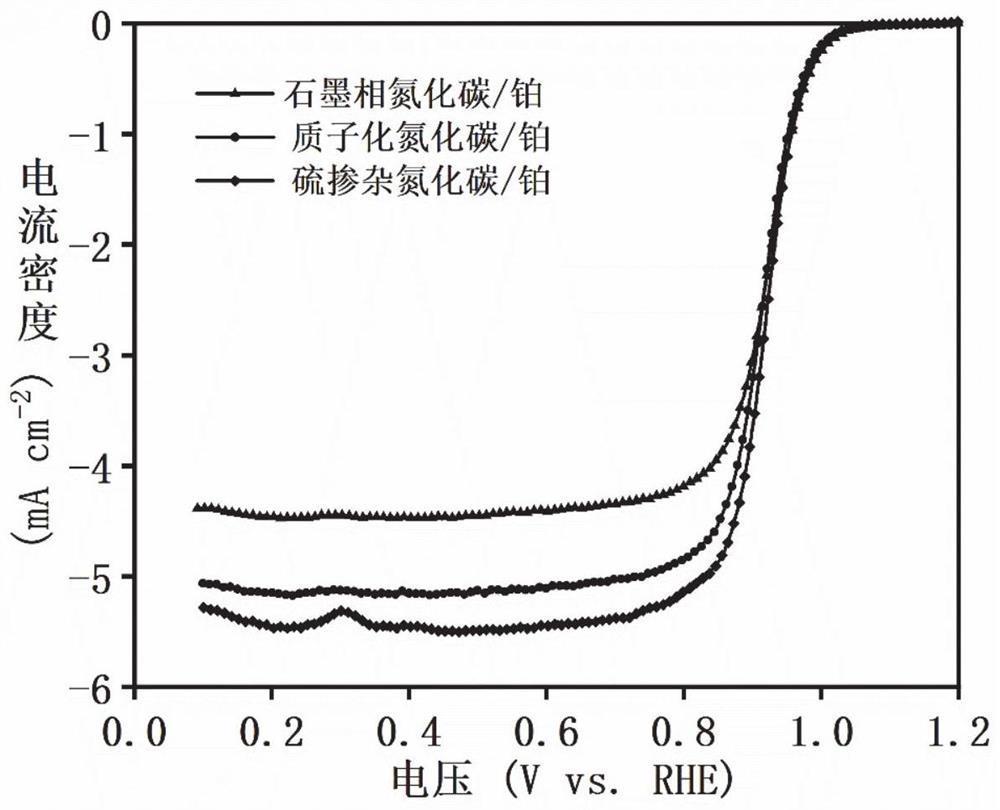
[0064] Step 1) Preparation method of modified carbon nitride: put 5g of melamine in a porcelain boat, put it in a tube furnace, raise the temperature to 550°C at a rate of 5°C / min, keep it warm for 3h, and drop it to room temperature to obtain graphite phase carbon nitride. Then take 1g of graphite phase carbon nitride and add 10ml of concentrated hydrochloric acid with a concentration of 4M, first ultrasonic for one hour, then magnetically stirred for three hours, after repeated washing with deionized water, the pH is close to 7, and the obtained product is protonated carbon nitride , placed in a blast oven to dry for 10 hours. Take 0.5g of protonated carbon nitride and 0.5g of sulfur powder and add them into a mortar for grinding. After grinding evenly, put the mixture of protonated carbon nitride and sulfur powder into a p...
Embodiment 2
[0070] A preparation method of a modified carbon nitride supported palladium oxygen reduction reaction electrocatalyst:
[0071] The method of this embodiment is basically the same as that of Embodiment 1, except that the supported noble metal is palladium. The specific steps include: the preparation method of the modified carbon nitride is the same as step 1) in Example 1. Dissolve 60mg of modified carbon nitride in a mixed solvent of 150ml of isopropanol and 200ml of deionized water, then add 0.2ml of an aqueous solution with a concentration of 0.1M potassium chloropalladate, ultrasonicate the resulting mixed solution for 1 hour and place it in an oil bath at 50°C Heated for 2 hours, after the reaction was completed, it was repeatedly washed with ethanol and water, centrifuged, and finally put into a vacuum oven for drying at 60°C. electrocatalyst.
[0072] In the present embodiment, the palladium particle diameter supported on the modified carbon nitride is about 10nm, su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com