Application of L-aspartic acid-beta-hydroxamate in preparation of medicine for treating type 2 diabetes
A technology of aspartic acid and hydroxamic acid, which can be used in drug combinations, metabolic diseases, pharmaceutical formulations, etc., can solve the problems of vitamin B12 deficiency, aggravating cognitive dysfunction in diabetic patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
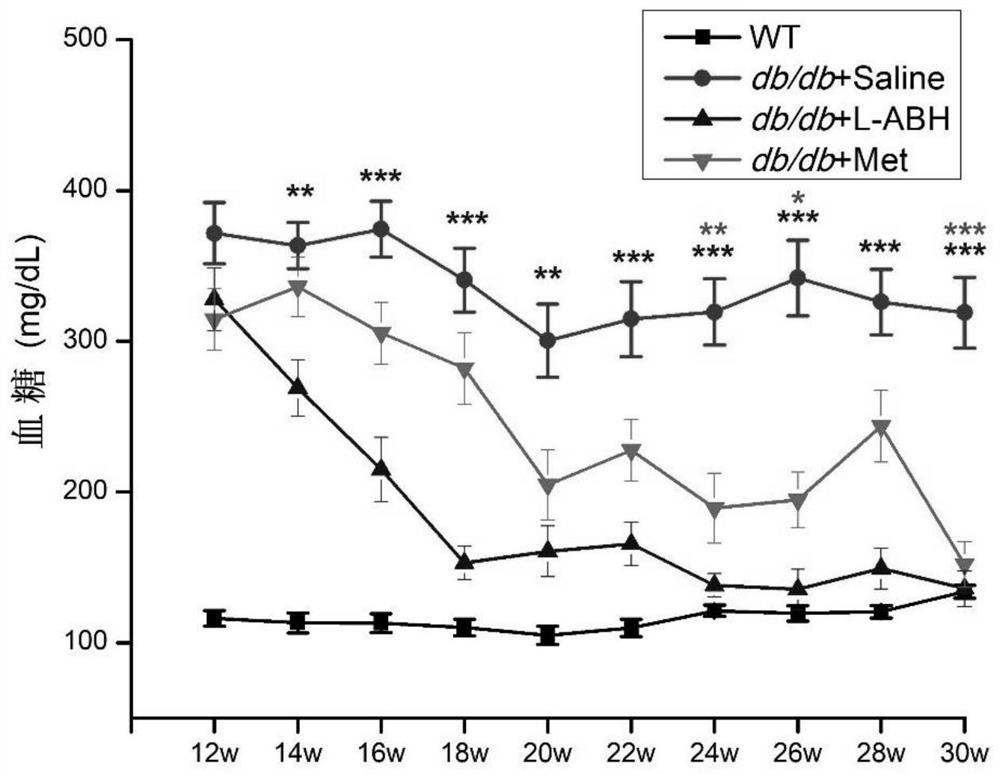
[0034] Several db / db mice were taken and divided into the following groups from the age of 12 weeks:
[0035] 1. Feed normal saline (denoted as db / db+saline, 0.06mL / 10g, 25 rats in total) from the age of 12;
[0036] 2. Feed Metformin (denoted as db / db+Met, 250mg / kg, 19 rats in total) from the age of 12;
[0037] 3. From the age of 12, LABH was fed (denoted as db / db+LABH, 20 mg / kg, 22 rats in total).
[0038] Each group was fed once a day according to the feeding dose for 18 weeks until the db / db mice were 30 weeks old.
[0039] Within one month after gavage, the wild-type mice (WT, gavage saline 0.06mL / 10g, 14) of the gavage group and the same age as db / db mice were checked once a week for random blood glucose, and the drug was gavaged for one month Afterwards, check random blood sugar every two weeks. The test results are shown in Table 1 and figure 1 as shown, figure 1 The statistical markers in the corresponding colors are the comparisons between the metformin group o...
Embodiment 2
[0044] Several WT mice and db / db mice were taken and divided into the following groups from the age of 12 weeks:
[0045] 1. Feed normal saline (denoted as db / db+saline, 0.06mL / 10g, 8 rats in total) from the age of 12;
[0046] 2. Feed Metformin (denoted as db / db+Met, 250mg / kg, 6 rats in total) from the age of 12;
[0047] 3. Feed LABH (denoted as db / db+LABH, 20mg / kg, 10 rats in total) from the age of 12;
[0048] 4. WT mice (10) did not receive any treatment.
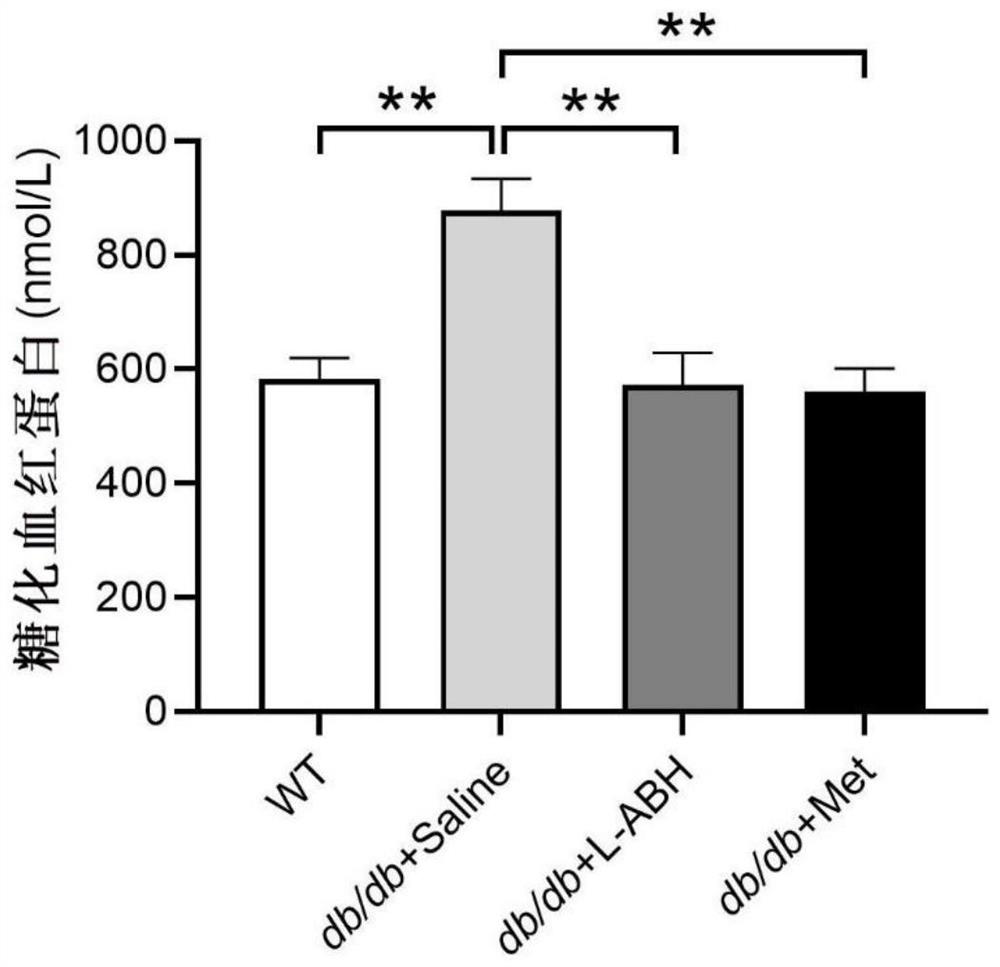
[0049] Once a day, fed for 18 weeks, continued until the 30th week of age. After the experiment, blood was taken to detect the content of glycosylated hemoglobin, and the test results are shown in Table 2 and figure 2 as shown, figure 2Medium **p<0.01.
[0050] Table 2 Effects of different drugs on glycosylated hemoglobin in db / db mice (nmol / L)
[0051] average value SEM WT 582.0842 37.37315 db / db+saline 877.9364 56.19169 db / db+LABH 572.363 56.28566 db / db+Met 561.2123 4...
Embodiment 3
[0054] Glucose Tolerance Test (OGTT)
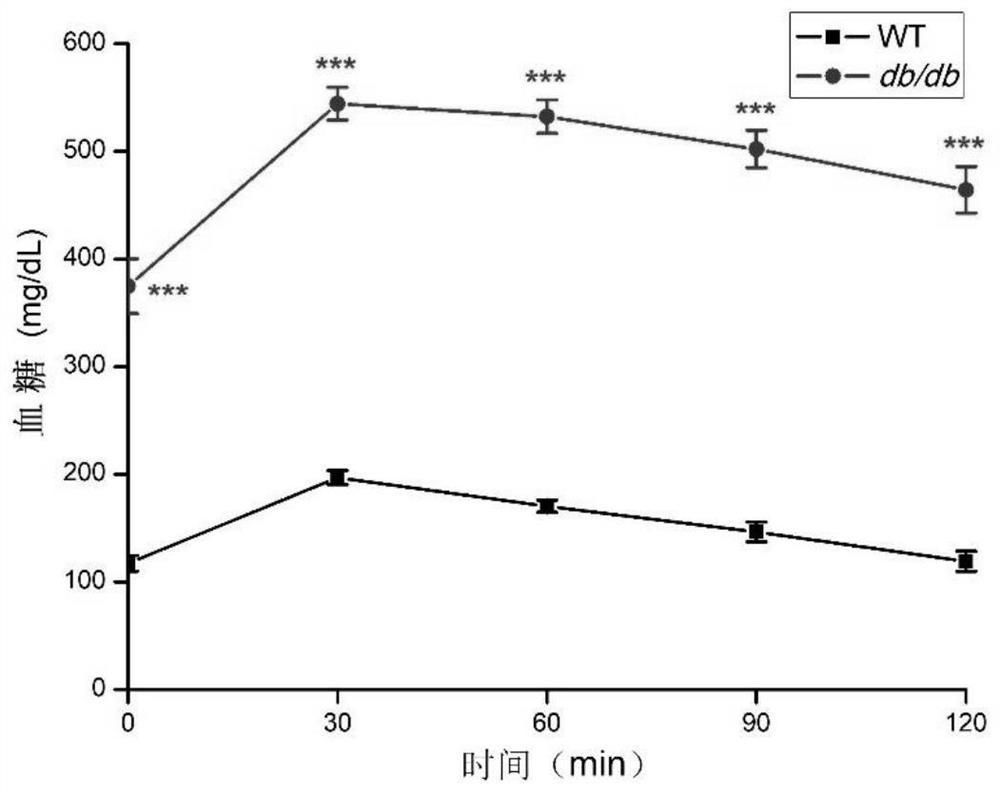
[0055] The 11-week-old WT mice and db / db mice were fed with glucose to detect the glucose tolerance of the mice. After fasting overnight, each mouse was fed with glucose 2g / kg. Blood glucose was detected in 30 minutes, 60 minutes, 90 minutes, and 120 minutes. The statistical results of the glucose tolerance of WT mice and db / db mice are shown in Table 3 and image 3 as shown, image 3 Moderate ***p<0.001.
[0056] Before table 3 administration, WT mice and db / db mice are to the degree of glucose tolerance (mg / dL)
[0057]
[0058] Several WT mice and db / db mice were taken and divided into the following groups from the age of 12 weeks:
[0059] 1. Feed normal saline (denoted as db / db+saline, 0.06mL / 10g, 21 rats in total) from the age of 12;
[0060] 2. Feed Metformin (denoted as db / db+Met, 250mg / kg, 20 rats in total) from the age of 12;
[0061] 3. Feed LABH (denoted as db / db+LABH, 20mg / kg, 22 rats in total) from the age of 12;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com