Reagent composition and application thereof for one-step screening and/or diagnosis of clonal diseases
A composition and reagent technology, applied in the field of blood disease detection, can solve the problems of low detection efficiency of rare tumors and precancerous lesions, heavy workload of the two-step method, and large individual differences in schemes, so as to facilitate the accumulation of data and experience, The effect of speeding up reporting time and reducing workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] The preparation of embodiment 1 reagent
[0095] The antibody combination used in this example is,
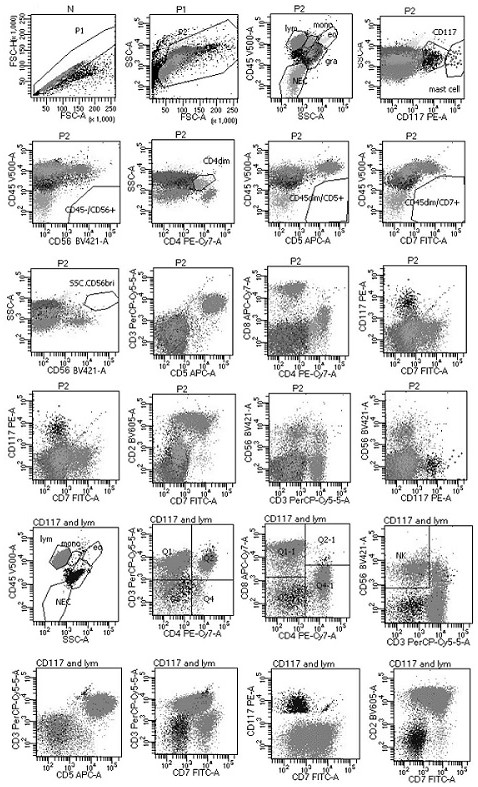
[0096] The first group is: the first group of antibodies includes: anti-CD7 antibody, anti-CD117 antibody, anti-CD3 antibody, anti-CD4 antibody, anti-CD5 antibody, anti-CD8 antibody, anti-CD56 antibody, anti-CD45 antibody and anti-CD2 antibody, each antibody The fluorescein labeling sequence is FITC, PE, PerCP-Cy5.5, PE-Cy7, APC, APC-Cy7, BV421, V500 and BV605; take the above nine monoclonal antibody reagents in a volume ratio of 5:5:5:3 : 2:3:3:3:3 volume ratio mixed in the first container;
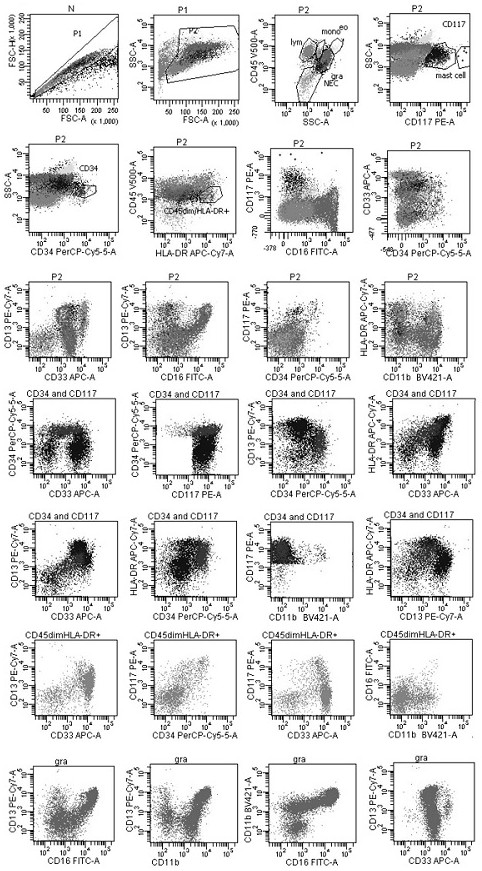
[0097] The second group is: anti-CD16 antibody, anti-CD117 antibody, anti-CD34 antibody, anti-CD13 antibody, anti-CD33 antibody, anti-HLA-DR antibody, anti-CD11b antibody and anti-CD45 antibody. The fluorescein labeling sequence of each antibody is FITC, PE, PerCP-Cy5.5, PE-Cy7, APC, APC-Cy7, BV421 and V500; the above eight monoclonal antibody reagents are mixed according to the ...
Embodiment 2
[0106] Example 2 Processing of Specimen
[0107] According to the results of cell counting, add heparin or EDTA anticoagulated bone marrow or peripheral blood samples into flow tube A, and ensure that the amount of cells added is about 2×10 6 According to Table 1, add 32 μl of nine kinds of cell membrane monoclonal antibody reagents labeled with different fluorescein to the flow tube, mix well with the cell suspension, incubate at room temperature and avoid light for 15 minutes, add 3ml 1× hemolysin, avoid Incubate with light for 10 minutes to lyse red blood cells, centrifuge at 1500rpm for 5 minutes to remove the supernatant, add 3ml of PBS to mix, centrifuge at 1500rpm for 5 minutes to remove the supernatant, add 0.5ml of PBS buffer to resuspend the cells, that is, the processed specimen, which can be used for On-board testing.
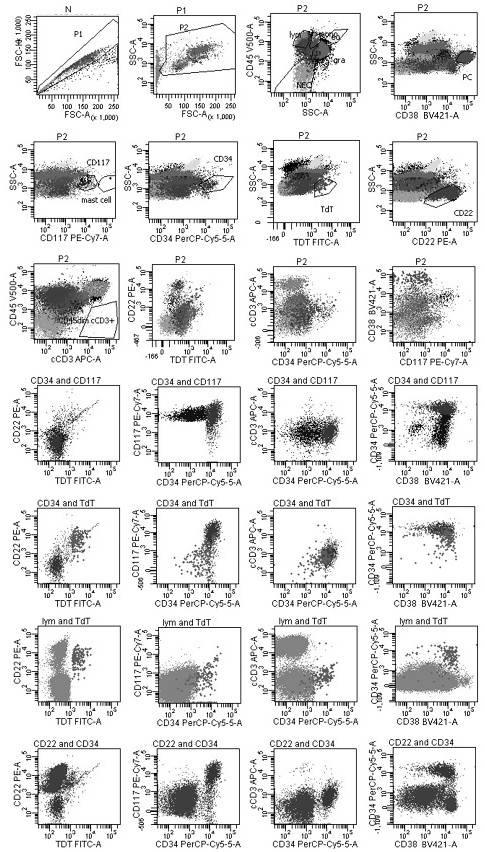
[0108] According to the results of cell counting, add heparin or EDTA anticoagulated bone marrow or peripheral blood samples into flow tube B, and...
Embodiment 3
[0114] Example 3 Detection of Specimen
[0115] The specimens processed according to the method of Example 2 were detected on the 3-laser 10-color FACS Canto plus flow cytometer of Becton Dickinson Company in the United States. After obtaining 1 million cells per tube (recommended at least 300,000), use diva 2.8 Software or other software such as Kaluza to analyze the data.
[0116] Among them, the flow cytometry gate is set in the following ways: ①Fixed gate: remove the adhesion cell gate, live cell gate, and blood cell gate in sequence; ②multi-marker combination gate: start from a single living cell, in order To prevent missing tumor cells, the gating and definition of all cells need to be carried out under parallel conditions with the blood cell gate; ③In the multi-marker combination gate, the common expression patterns and development patterns of various marker combinations are displayed, based on the normal Different cells, to find tumor cells; or naive T cells or naive ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com