A kind of bistable electrochromic fluorane dye and preparation method thereof
An electrochromic device and fluoran dye technology, applied in the field of material science, can solve the problem that the color development state can only be maintained for a few minutes, and achieve the effect of improving the electrochromic response rate and excellent bistable characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
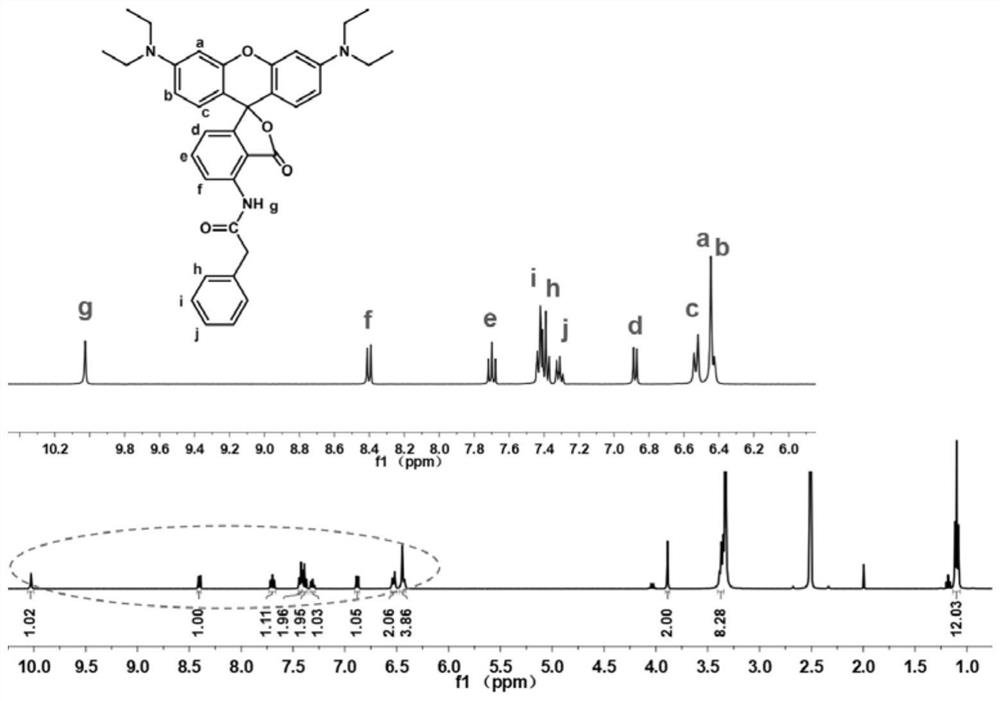
[0042] Preparation of red No. 3 novel fluoran dye: 50mmol 3-(diethylamino)phenol and 25mmol 3-nitrophthalic anhydride were added to a three-necked flask containing 50mL of chlorobenzene, after complete dissolution, 25mmol of trifluoride was added Methanesulfonic acid. Then, the three-necked flask was placed in a 135° C. oil bath to preheat, and the reaction was carried out under nitrogen reflux for 2 days. After the reaction was completed, it was cooled to room temperature, and the solvent was removed by rotary evaporation. Column chromatography was carried out with dichloromethane (DCM) / methanol as the eluent to obtain a purple-red powder, namely the product ph-NO 2 .
[0043] 1mmol ph-NO 2 , 20 mg of Pd / C, 4 mL of ethyl acetate (EtOAc) were placed in a three-necked flask. 1.5mmol H 3 PO 2 and 4.5mmol NaH 2 PO 2 ·H 2 O is homogeneously dissolved in 4 mL of H 2 After O, poured into the above three-necked flask. Then, the three-necked flask was placed in an oil bath a...
Embodiment 2
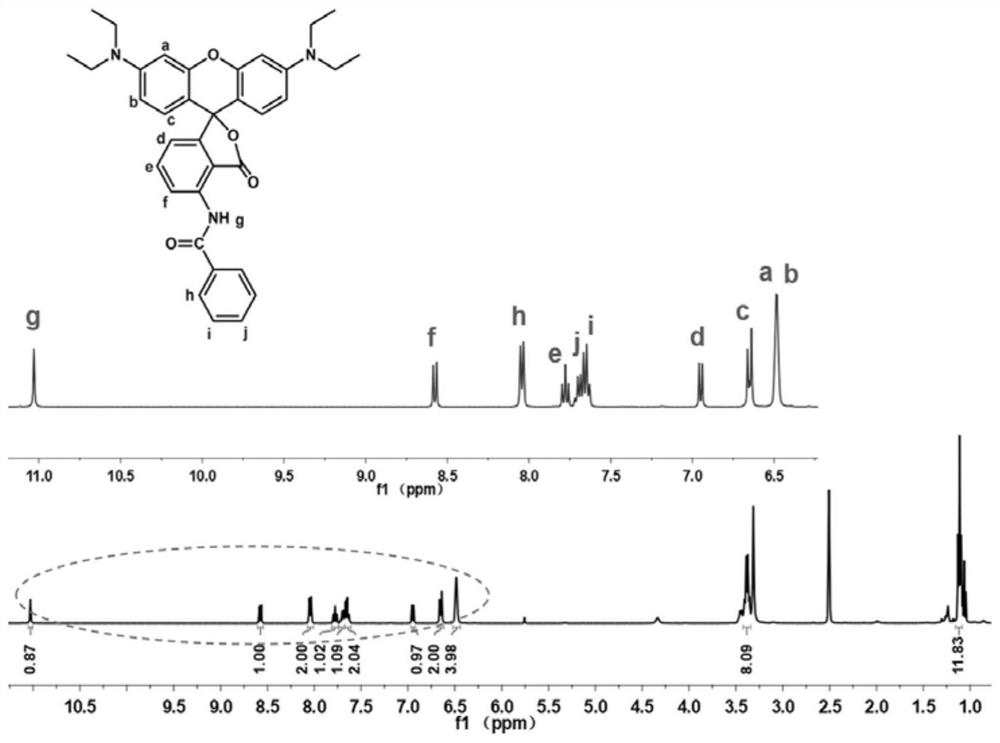
[0064] Preparation of red No. 4 novel fluoran dye: 50mmol of 3-(diethylamino)phenol and 25mmol of 3-nitrophthalic anhydride were added to a three-necked flask containing 50mL of chlorobenzene, and after completely dissolving, 25mmol of trifluoride was added Methanesulfonic acid. Then, the three-necked flask was placed in a 135° C. oil bath to preheat, and the reaction was carried out under nitrogen reflux for 2 days. After the reaction was completed, it was cooled to room temperature, and the solvent was removed by rotary evaporation. Column chromatography was carried out with dichloromethane (DCM) / methanol as the eluent to obtain a purple-red powder, namely the product ph-NO 2 .
[0065] 1mmol ph-NO 2 , 20 mg of Pd / C, 4 mL of ethyl acetate (EtOAc) were placed in a three-necked flask. 1.5mmol H 3 PO 2 and 4.5mmol NaH 2 PO 2 ·H 2 O is homogeneously dissolved in 4 mL of H 2 After O, poured into the above three-necked flask. Then, the three-necked flask was placed in an...
Embodiment 3
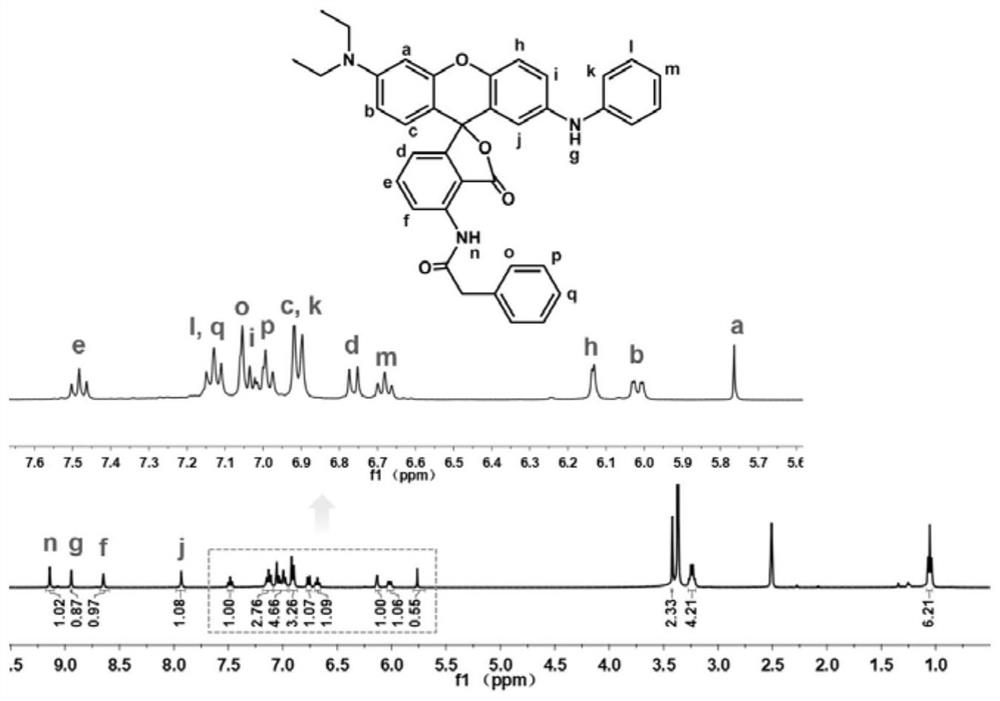
[0074] Preparation of Green No. 3 Novel Fluoran Dye: Dissolve 3-diethylaminophenol (1.65g) and 3-nitrophthalic anhydride (2.89g) in toluene (25ml), put into a three-necked The reaction was carried out in a bath for 4 h, cooled to room temperature, and extracted with a small amount of petroleum ether. The supernatant was removed to obtain a purple-red precipitate. The precipitate was purified by column chromatography using dichloromethane / methanol as the eluent to obtain a golden yellow powder, which was the product 4-diethylaminonitroketo acid.
[0075] 4-Diethylamino nitroketo acid (intermediate M1) (2 g) and concentrated sulfuric acid (15 ml) were put into a three-necked flask, and the powder was dissolved in an ice-water bath. Then 4-methoxydiphenylamine (1.11 g) was put into the above three-necked flask, and the reaction was carried out at room temperature for 24 h. After that, the product was slowly dropped into ice water to obtain a dark green precipitate, which was wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com