Bistable electrochromic fluorane dye and preparation method of bistable electrochromic fluorane dye device
A technology of electrochromic devices and fluorane dyes, which is applied in the field of material science, can solve the problem that the color state can only be maintained for a few minutes, and achieve the effect of improving the response rate of electrochromism and excellent bistable characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
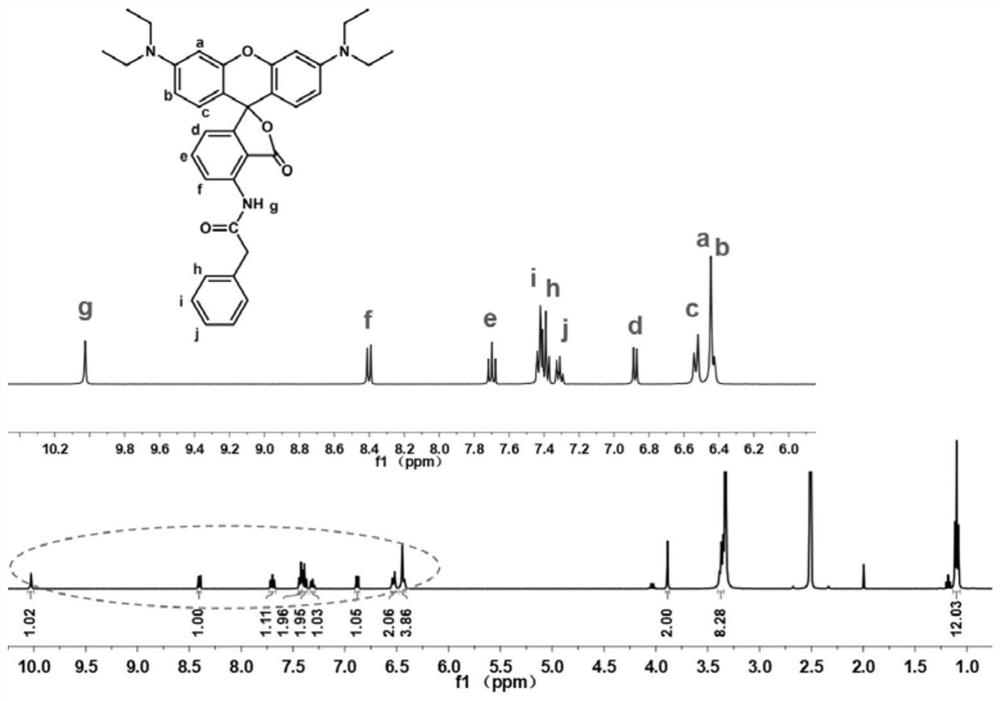
[0042] Preparation of red No. 3 new fluoran dye: 50mmol 3-(diethylamino)phenol and 25mmol 3-nitrophthalic anhydride were added to a three-necked flask containing 50mL chlorobenzene, after they were completely dissolved, 25mmol trifluoro Methanesulfonic acid. Then put the three-neck flask into a 135°C oil bath to preheat, and react under nitrogen reflux for 2 days. After the reaction is completed, cool to room temperature, and remove the solvent by rotary evaporation. Using dichloromethane (DCM) / methanol as eluent for column chromatography, a purple powder was obtained, the product ph-NO 2 .
[0043] 1 mmol ph-NO 2 , 20mg Pd / C, 4mL ethyl acetate (EtOAc), put into a three-necked flask. 1.5 mmol H 3 PO 2 and 4.5 mmol NaH 2 PO 2 ·H 2 O was homogeneously dissolved in 4 mL of H 2 After being in O, it was poured into the above-mentioned three-neck flask. Then the three-neck flask was put into an oil bath at 85°C for 5 hours, cooled to room temperature, and extracted with di...
Embodiment 2
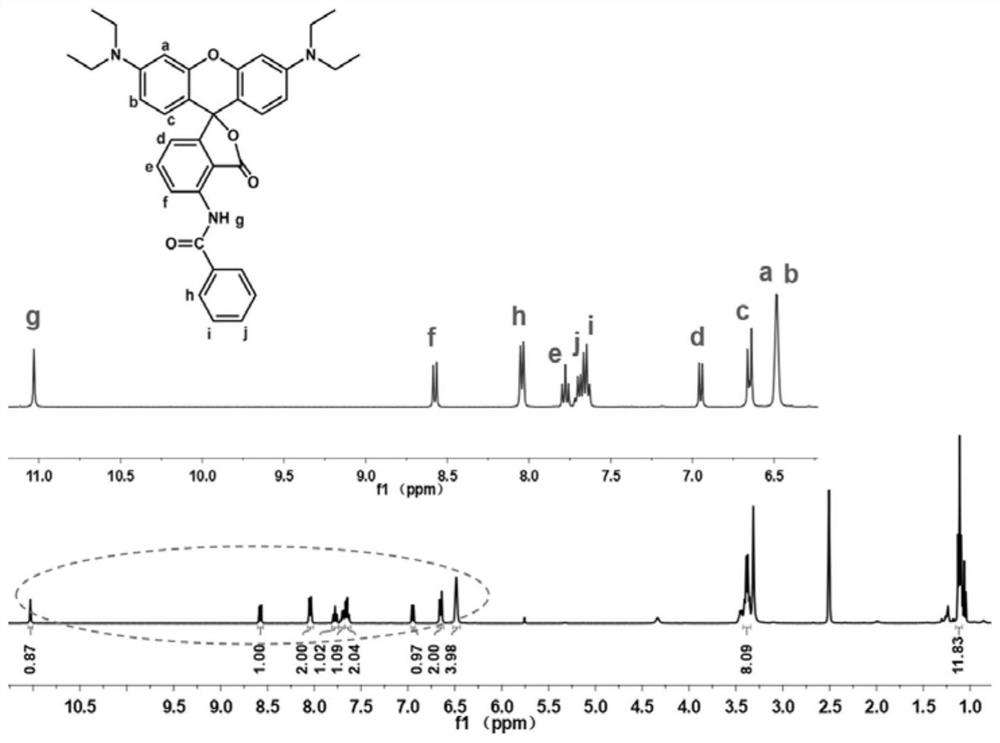
[0064] Preparation of red No. 4 novel fluoran dye: 50mmol 3-(diethylamino)phenol and 25mmol 3-nitrophthalic anhydride were added to a three-necked flask containing 50mL chlorobenzene, after they were completely dissolved, 25mmol trifluoro Methanesulfonic acid. Then put the three-neck flask into a 135°C oil bath to preheat, and react under nitrogen reflux for 2 days. After the reaction is completed, cool to room temperature, and remove the solvent by rotary evaporation. Using dichloromethane (DCM) / methanol as eluent for column chromatography, a purple powder was obtained, the product ph-NO 2 .
[0065] 1 mmol ph-NO 2 , 20mg Pd / C, 4mL ethyl acetate (EtOAc), put into a three-necked flask. 1.5 mmol H 3 PO 2 and 4.5 mmol NaH 2 PO 2 ·H 2 O was homogeneously dissolved in 4 mL of H 2 After being placed in O, it was poured into the above-mentioned three-neck flask. Then the three-neck flask was put into an oil bath at 85°C for 5 hours, cooled to room temperature, and extracte...
Embodiment 3
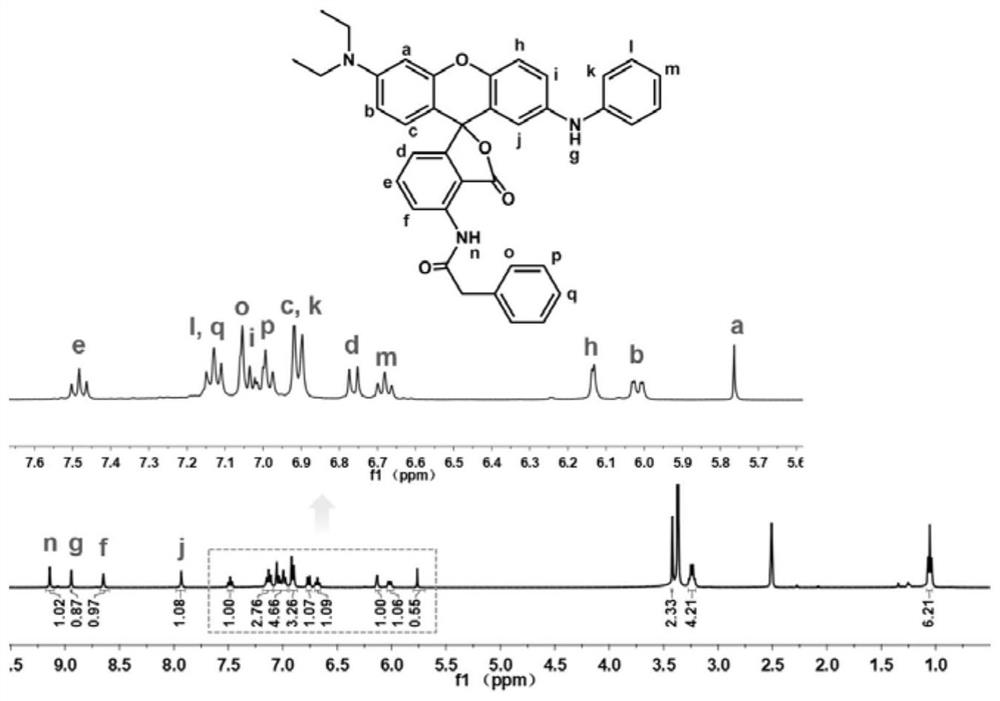
[0074] Preparation of Green No. 3 novel fluoran dye: 3-diethylaminophenol (1.65g) and 3-nitrophthalic anhydride (2.89g) were dissolved in toluene (25ml), put into a three-necked flask, 110°C oil The reaction was carried out in a bath for 4h, and after cooling to room temperature, it was extracted with a small amount of petroleum ether. The supernatant was removed to obtain a purple-red precipitate. Purify the precipitate by column chromatography using dichloromethane / methanol as the eluent to obtain a golden yellow powder, which is the product 4-diethylaminonitroketoacid.
[0075] Put 4-diethylaminonitroketo acid (intermediate M1) (2g) and concentrated sulfuric acid (15ml) into a three-neck flask, and dissolve the powder in an ice-water bath. Then, 4-methoxydiphenylamine (1.11 g) was put into the above-mentioned three-necked flask, and reacted at room temperature for 24 h. Afterwards, the product was slowly dropped into ice water to obtain a dark green precipitate, which was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com