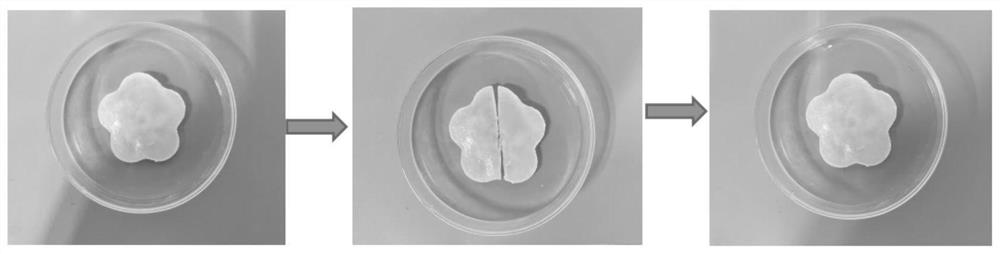
Self-healing hydrogel phase-change material and preparation method thereof
A technology of phase change materials and inorganic phase change materials, which is applied in the field of self-healing hydrogel phase change materials and its preparation, can solve the problems of inability to protect the phase change materials and poor mechanical properties of hydrogels, and achieve the elimination of phase change materials. Effects of separation phenomenon, high latent heat of phase change, and high thermal conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) take by weighing 40g sodium sulfate decahydrate, melt under the airtight situation of 40 degrees centigrade in water bath;
[0039] (2) Weigh 1.6g of borax, 0.04g of sodium hexametaphosphate and 1.2g of sodium acrylate, and pour them into molten sodium sulfate decahydrate solution successively, keep the temperature of the water bath at 40 degrees centigrade and heat tightly, and stir until the solution is evenly dispersed;
[0040] (3) Add 0.2g of N,N-methylenebisacrylamide, 0.08g of sodium alginate, and 0.4g of calcium sulfate dihydrate to the dispersion obtained in step (2), heat the water bath at 40 degrees Celsius, and stir until the solution is evenly dispersed (Continue to stir for about 20 minutes until the solution is evenly stirred and no stratification occurs);
[0041] (4) Pour 0.6g initiator ammonium persulfate into 4g of deionized water, after fully dissolving, add the ammonium persulfate initiator solution drop by drop, during which the heating tempera...
Embodiment 2
[0046] (1) Take by weighing 40g disodium hydrogen phosphate dodecahydrate, melt under the airtight situation of 45 degrees centigrade in water bath;
[0047] (2) Weigh 1.6g of sodium citrate dihydrate, 0.04g of sodium hexametaphosphate and 1.2g of sodium acrylate, and pour them into molten disodium hydrogen phosphate dodecahydrate solution successively, keep the water bath temperature at 45 degrees Celsius, and stir until The solution is evenly dispersed;
[0048] (3) Add 0.2g of N,N-methylenebisacrylamide, 0.08g of sodium alginate, and 0.4g of calcium sulfate dihydrate to the dispersion obtained in step (2), keep the temperature of the water bath at 45 degrees Celsius and heat, and stir until the solution is evenly dispersed (Continue to stir for about 20 minutes until the solution is evenly stirred and no stratification occurs);
[0049] (4) Pour 0.6g initiator potassium persulfate into 4g deionized water, after fully dissolving, add potassium persulfate initiator solution ...
Embodiment 3
[0054] (1) take by weighing 40g sodium acetate trihydrate, melt under the airtight situation of 58 degrees centigrade in water bath;
[0055] (2) Weigh 2g of disodium hydrogen phosphate dodecahydrate, 0.04g of sodium hexametaphosphate and 1.2g of chitosan and pour them into molten sodium acetate trihydrate solution successively, keep the temperature of the water bath at 58 degrees Celsius, and stir until the solution Evenly dispersed;
[0056] (3) Add 0.2g of N,N-methylenebisacrylamide, 0.08g of sodium alginate, and 0.4g of calcium sulfate dihydrate to the dispersion obtained in step (2), keep the temperature of the water bath at 58 degrees Celsius and heat, and stir until the solution is evenly dispersed (Continue to stir for about 20 minutes until the solution is evenly stirred and no stratification occurs);
[0057] (4) Add 1.2g of photoinitiator 1173 dropwise to the above solution, and then add 4g of deionized water, during which the heating temperature of the water bath ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Latent heat of phase change | aaaaa | aaaaa |
| Thermal conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com