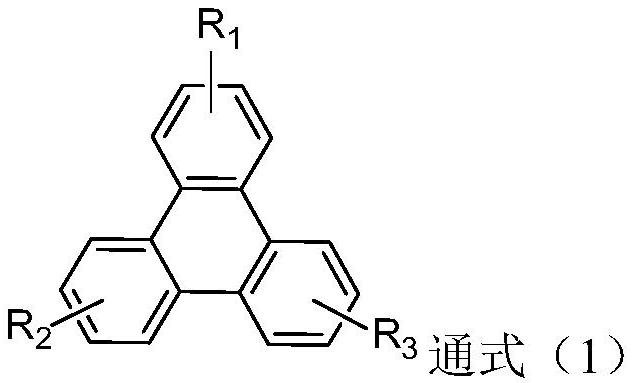
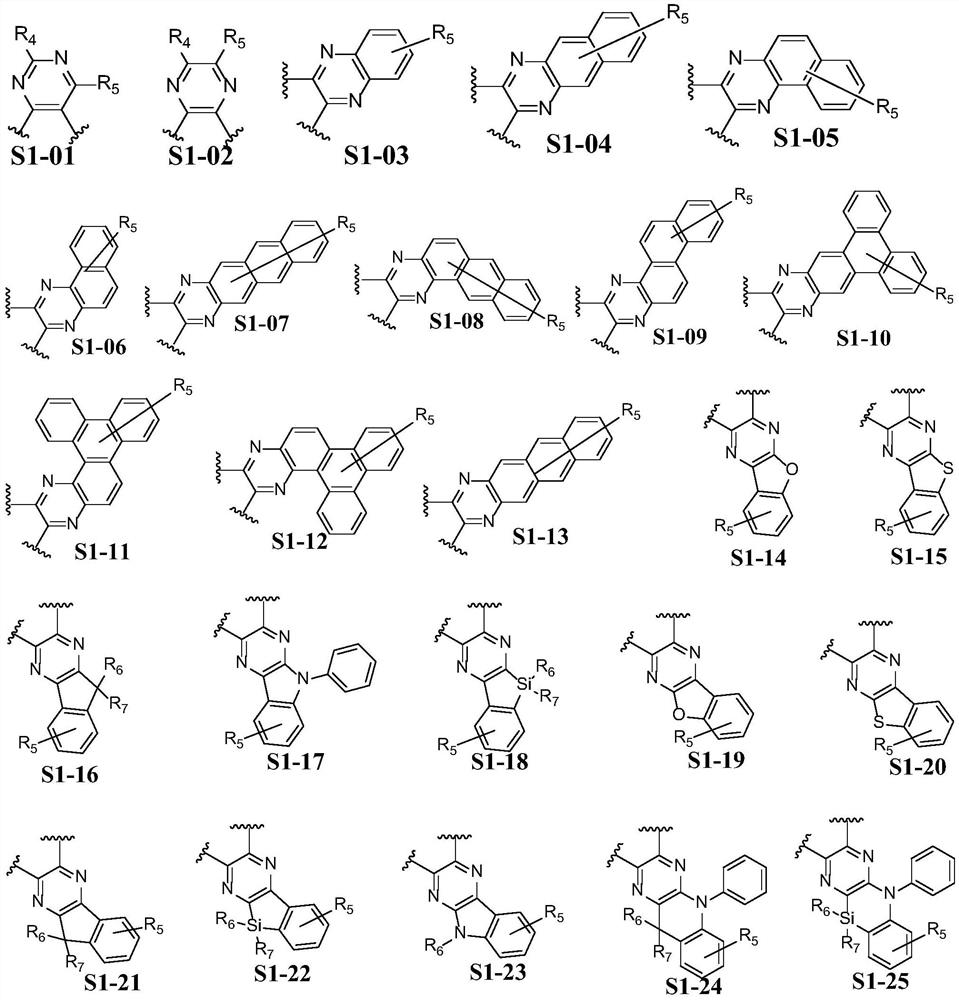
Triphenylene derivative, light-emitting element material and light-emitting element
A derivative, triphenylene technology, applied to triphenylene derivatives, can solve problems such as lack of materials, and achieve the effects of easy availability of raw materials, high-efficiency luminescence performance, and reduction of vibration energy loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Synthesis of compound 1
[0068]
[0069] Under an argon atmosphere, 50.5 grams (100 mmol) of N-(7,10-dibromoterphenyl-2-yl) benzamide and 12.5 grams (120 mmol) of 2-chloropyridine were added to the reaction flask, and 300 ml of methylene chloride was cooled to -78°C, then 31.0 g (110 mmol) of trifluoromethanesulfonic anhydride was added, and the reaction mixture was placed in an ice-water bath and heated to 0°C. 11 g (110 mmol) of benzonitrile was added. The reaction mixture was heated to 45°C and reacted for 6 hours, then cooled to room temperature and triethylamine was added to neutralize trifluoromethanesulfonate. The volatiles were removed under reduced pressure, and 51.9 g of 3,6-dibromo-11,13-diphenylphenanthroquinazoline were obtained by flash column chromatography (eluent: 10% ethyl acetate in hexane), Yield 88%, HPLC purity 99.6%.
[0070] 1 HNMR(DMSO): δ9.10(s, 1H), 8.93(s, 1H), 8.81(d, 1H), 8.35(m, 2H), 8.29(s, 1H), 8.12(s, 1H), 8.05 ~7.99(m, 3H), 7.8...
Embodiment 2
[0074] Synthesis of compound 20
[0075]
[0076] Under an argon atmosphere, 50.5 grams (100 mmol) of N-(7,10-dibromoterphenyl-2-yl) benzamide and 12.5 grams (120 mmol) of 2-chloropyridine were added to the reaction flask, and 300 ml of methylene chloride was cooled to -78°C, then 31.0 g (110 mmol) of trifluoromethanesulfonic anhydride was added, and the reaction mixture was placed in an ice-water bath and heated to 0°C. 11 g (110 mmol) of benzonitrile was added. The reaction mixture was heated to 45°C and reacted for 6 hours, then cooled to room temperature and triethylamine was added to neutralize trifluoromethanesulfonate. The volatiles were removed under reduced pressure, and 51.9 g of 3,6-dibromo-11,13-diphenylphenanthroquinazoline were obtained by flash column chromatography (eluent: 10% ethyl acetate in hexane), Yield 88%, HPLC purity 99.6%.
[0077] 1 HNMR(DMSO): δ9.10(s, 1H), 8.93(s, 1H), 8.81(d, 1H), 8.35(m, 2H), 8.29(s, 1H), 8.12(s, 1H), 8.05 ~7.99(m, 3H), 7....
Embodiment 3
[0082] Synthesis of Compound 28
[0083]
[0084] Under an argon atmosphere, add 46.0 grams (100 mmol) of N-(7-bromo-10-chlorobenzo-2-yl) benzamide and 12.5 grams (120 mmol) of 2-chloropyridine to 300 ml of dichloromethane in the reaction flask, and cool down to -78°C, then added 31.0 g (110 mmol) of trifluoromethanesulfonic anhydride, and placed the reaction mixture in an ice-water bath and heated to 0°C. Added 11 g (110 mmol) of benzonitrile. The reaction mixture was heated to 45°C and reacted for 6 hours, then cooled to room temperature and triethylamine was added to neutralize trifluoromethanesulfonate. The volatiles were removed under reduced pressure and flash column chromatography (eluent: 10% ethyl acetate in hexane) gave 46.3 g of 6-bromo-3-chloro-11,13-diphenylphenanthroquinazoline , yield 85%, HPLC purity 99.4%.
[0085] 1 HNMR(DMSO): δ9.10(s, 1H), 8.93(s, 1H), 8.81(d, 1H), 8.35(m, 2H), 8.29(s, 1H), 8.12(s, 1H), 8.05 ~7.99(m, 3H), 7.80(d, 2H), 7.65(t, 2H), 7....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com