Insulin analogs having reduced insulin receptor binding affinity
An insulin analog, insulin technology, applied in the directions of insulin, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
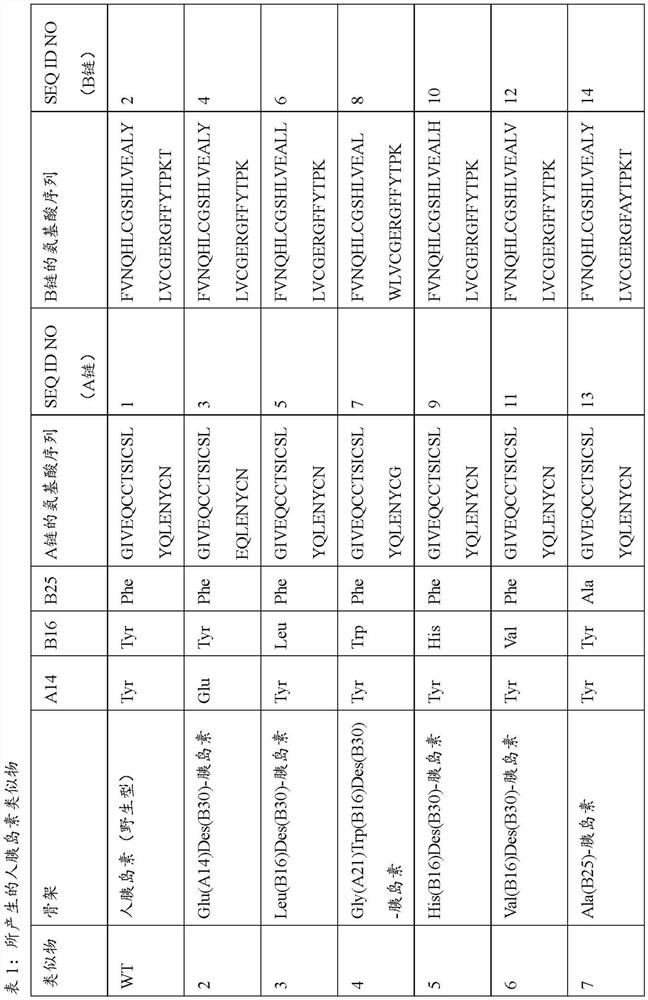
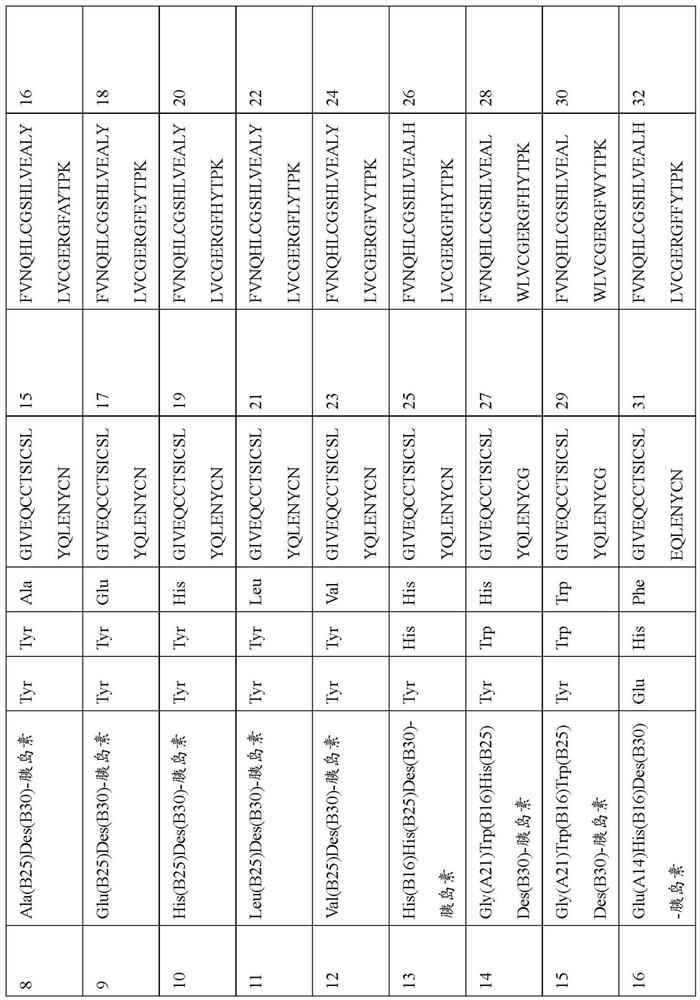
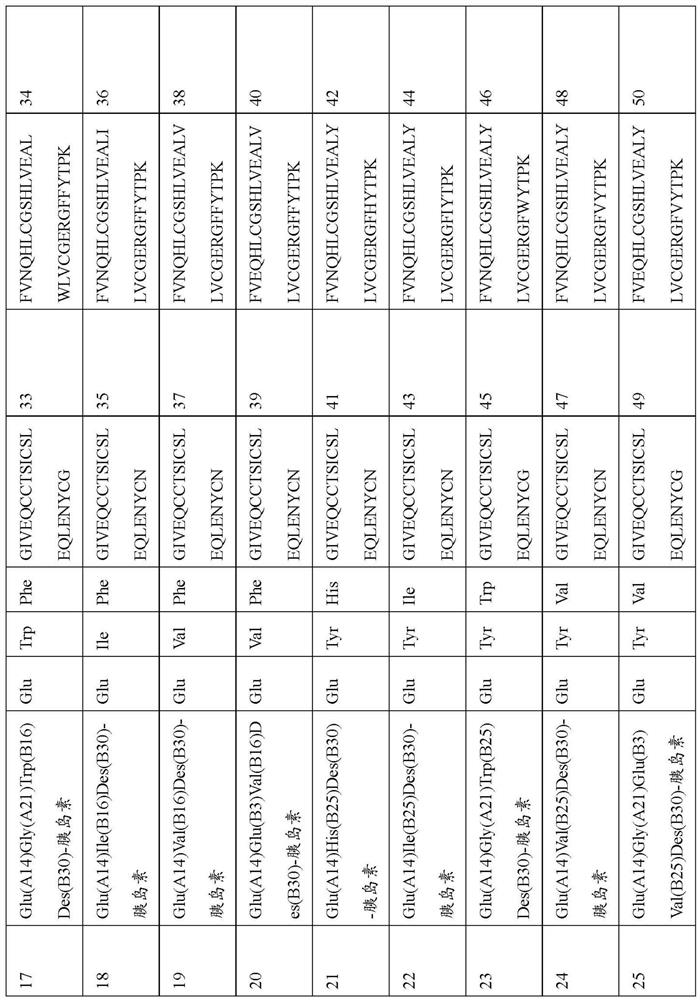
[0329] Example 1: Production of Human Insulin and Insulin Analogs
[0330] Recombinant production of human insulin as well as insulin analogs. The polynucleotide encoding preproinsulin is derived from order. The designed polynucleotides were optimized for expression in yeast. They were inserted into expression vectors by classical restriction cloning, enabling functional expression and secretion in K. lactis K. As a secretion leader, the gene was C-terminally fused to a DNA sequence encoding the alpha mating factor signal of Saccharomyces cerevisiae. Recombinant gene expression was controlled by a lactose-inducible K. lactis promoter.
[0331] Human insulin and insulin analogs are manufactured as preproinsulin. A genetically fused N-terminal pre-sequence is used to improve expression and secretion yields and to stabilize the peptide in culture broth. A wide variety of sequences can be used for this purpose and tested for efficiency. Proinsulin itself consists of a B-ch...
Embodiment 2
[0342] Example 2: Insulin Receptor Binding Affinity Determination / Insulin Receptor Autophosphorylation Determination
[0343] Insulin binding and signaling of the various insulin analogs produced were determined by binding assays and receptor autophosphorylation assays.
[0344] A) Insulin receptor binding affinity determination
[0345] The insulin receptor binding affinities of the analogs listed in Table 1 were determined as described by Hartmann et al. (Effect of the long-acting insulin analogs glargine and degludec on cardiomyocyte cell signaling and function. Cardiovasc Diabetol. 2016; 15:96). Isolation and competition binding experiments of the plasma membrane embedded insulin receptor (M-IR) were performed as previously described (Sommerfeld et al., PLoS One. 2010;5(3):e9540). Briefly, CHO cells overexpressing IR were collected and resuspended in ice-cold 2.25STM buffer (2.25M sucrose, 5mM Tris-HCl pH7.4, 5mM MgCl 2 , complete protease inhibitors) and disrupted usin...
Embodiment 3
[0361] Example 3: Determination of in vitro stability in different recombinant proteases and simulated gastric juice
[0362] The proteolytic stability of insulin analogues was tested (α-chymotrypsin, cathepsin D, insulin-degrading enzyme (IDE) and simulated gastric juice.
[0363] A) Measurement conditions
[0364]
[0365] B) Preparation of simulated gastric juice
[0366] Two grams of sodium chloride and 3.2 g of purified pepsin (from porcine gastric mucosa, activity 800 to 2500 units / mg protein) were dissolved in 7.0 ml of hydrochloric acid. The volume was adjusted with water up to 1000ml. The resulting solutions were mixed and adjusted to a pH of 1.2±0.1 with 0.2N sodium hydroxide or 0.2N hydrochloric acid.
[0367] C) General Assay Procedure
[0368] Stability determinations were performed using appropriate time points (15, 30, 60, 120 and 240 minutes for SIF and SGF; 15, 30, 60 and 120 minutes for proteases). Incubation was performed at 37°C and the % pare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com