Co-loaded liposome based on simultaneous killing of tumor cells and CAFs and preparation method of co-loaded liposome
A tumor cell and liposome technology is applied in the field of co-loaded liposomes based on simultaneous killing of tumor cells and CAFs and their preparation, which can solve the problems of patient death, tumor metastasis and recurrence, and tumor treatment failure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
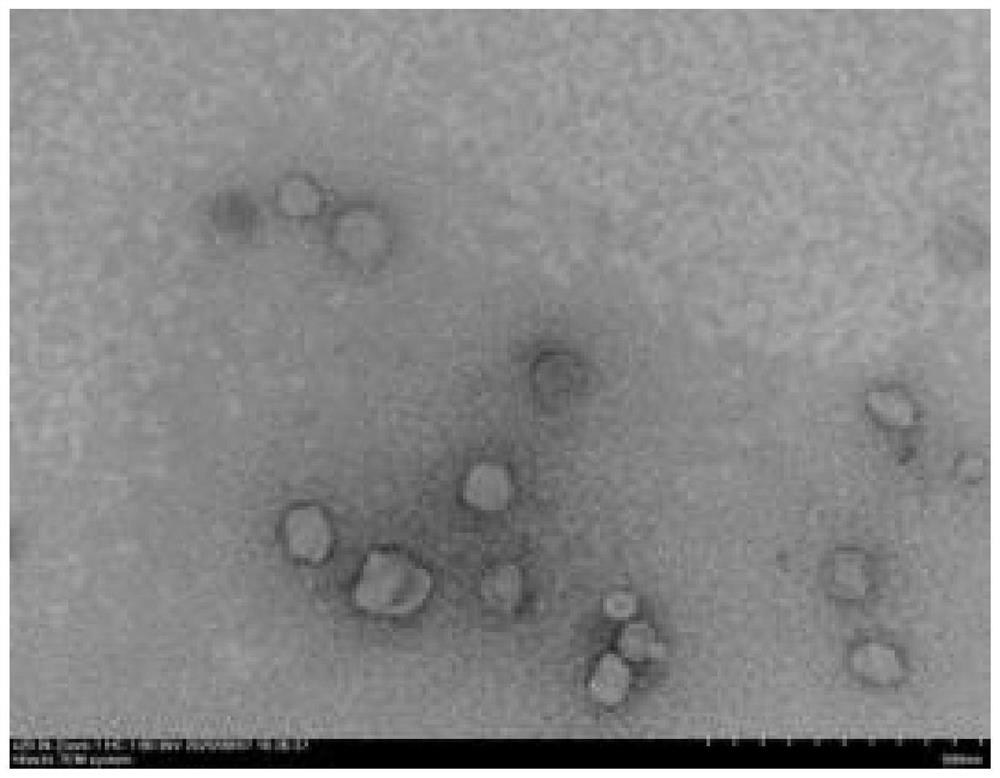
[0032] A co-loaded liposome based on simultaneous killing of tumor cells and CAFs, the co-loaded liposome is co-loaded irinotecan / anti-FAP scFv liposome, prepared from the following components: egg yolk lecithin 95mg , Cholesterol 25mg, DSPE-PEG-R927mg, Irinotecan 15mg, anti-FAP scFv (a kind of single-chain monoclonal antibody, its mass ratio to DSPE-PEG-R9 is 1:540).
[0033] The preparation method of the above-mentioned co-loaded liposomes comprises the following steps: first, dissolve the weighed egg yolk lecithin, cholesterol, DSPE-PEG-R9, and irinotecan in 2mL absolute ethanol, at a speed of 20rpm / min, Inject into 5mL PBS at a temperature of 60°C, and obtain positively charged liposomes after 1 hour; then mix the anti-FAP scFv with the positively charged liposomes and vortex for 15 minutes, pass through 450nm and 200nm filters three times each, Liposomes co-loaded with irinotecan / anti-FAP scFv were obtained.
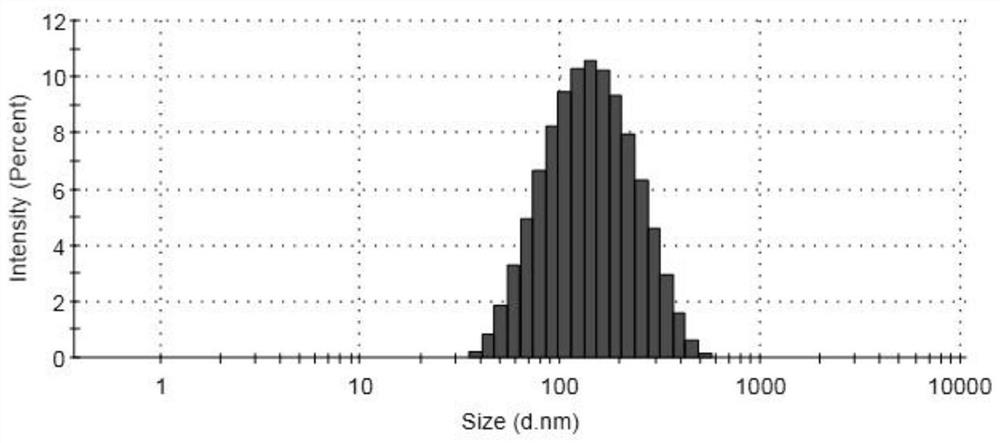
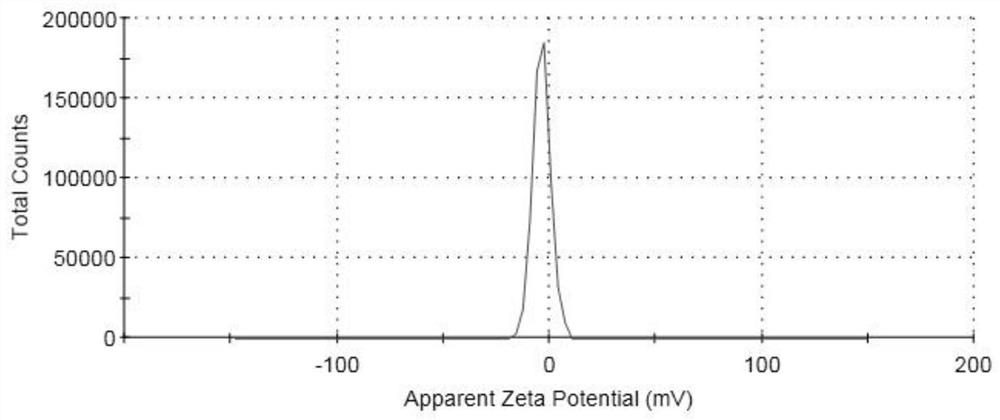
[0034] After testing, the concentration of irinotecan in the ...
Embodiment 2
[0039] A co-loaded liposome based on simultaneous killing of tumor cells and CAFs, the co-loaded liposome is a co-loaded doxorubicin / anti-FAP scFv liposome, prepared from the following components: soybean lecithin 95mg , Cholesterol 25mg, DSPE-PEG-R927mg, Doxorubicin 5mg, anti-FAP scFv (a kind of single-chain monoclonal antibody, its mass ratio to DSPE-PEG-R9 is 1:270).
[0040]The preparation method of the above co-loaded liposomes comprises the following steps: first, dissolve the weighed soybean lecithin, cholesterol, DSPE-PEG-R9, and doxorubicin in 6 mL of chloroform, and add 2 mL of PBS solution for a short time. Sonicate to form a water-in-oil emulsion, use a rotary evaporator to remove chloroform to form a gel, then add an appropriate amount of PBS to 5mL, and hydrate at 60°C for 1 hour to obtain positively charged liposomes; then anti -FAP scFv was mixed with the positively charged liposome and vortexed for 15 minutes, and passed through 450 nm and 200 nm filter membra...
Embodiment 3
[0043] A co-loaded liposome based on simultaneous killing of tumor cells and CAFs, the co-loaded liposome is a co-loaded Sorafenib / Sirolizumab liposome, prepared from the following components: hydrogenated soybean Phospholipids 95mg, Cholesterol 25mg, DOTAP 4.5mg, Sorafenib 15mg, Cirocizumab (a kind of monoclonal antibody drug, its mass ratio to DOTAP is 1:540).
[0044] The preparation method of the above-mentioned co-loaded liposomes comprises the following steps: first, dissolve the weighed hydrogenated soybean lecithin, cholesterol, DOTAP, and Sorafenib in 4mL chloroform / methanol mixed solution, and use a rotary evaporator to vacuumize and remove Organic solvents were used to obtain a uniform lipid film, and then 5 mL of PBS solution was added to hydrate at 60°C for 1 hour to obtain positively charged liposomes; then the positively charged liposomes were mixed with cilozumab and the above positively charged liposomes were vortexed for 15 min, passed through 450 nm and 200n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com