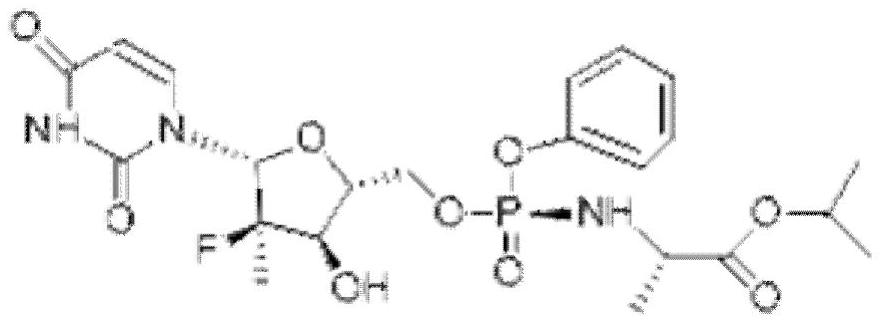
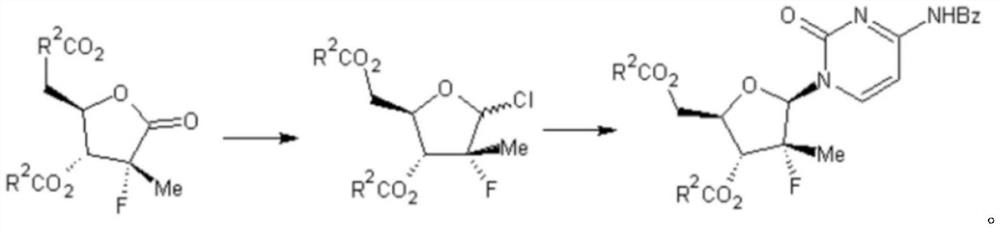
Preparation method of sofosbuvir intermediate
A technology for intermediates and compounds, applied in the field of pharmaceuticals, can solve the problems of excessive reduction, generation of by-products, inconvenient reaction, etc., and achieve the effects of improving product purity, reducing by-product generation, and improving purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2~5
[0058] According to the method disclosed in Example 1, only the feeding ratio of compound a-1 and 70% red aluminum in the preparation of compound b-1 from compound a-1 was changed, and the results are shown in Table 1.
[0059] Table 1 Different reaction conditions and results
[0060] Example no a-1 :n 红铝
Embodiment 6~16
[0062] According to the method disclosed in Example 1, only the chlorinated reagent, the feed ratio of compound b-1 and the chlorinated reagent, and the reaction temperature in the operation of preparing compound c-1 by chlorinated reaction of compound b-1 were changed respectively. The results are shown in the table 2.
[0063] Note: blank spaces in the table indicate that this parameter is the same as in Example 1.
[0064] Table 2 Different reaction conditions and results
[0065]
[0066]
Embodiment 17~24
[0068] According to the method disclosed in Example 1, only the feed ratio and reaction temperature of compound c-1 and e-1 in the operation of preparing target compound d-1 with compound c-1 as substrate were changed respectively. The results are shown in Table 3.
[0069] Note: blank spaces in the table indicate that this parameter is the same as in Example 1.
[0070] Table 3 Different reaction conditions and results
[0071]
[0072]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com