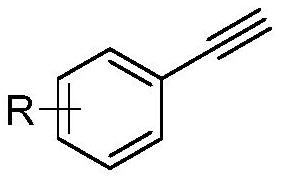
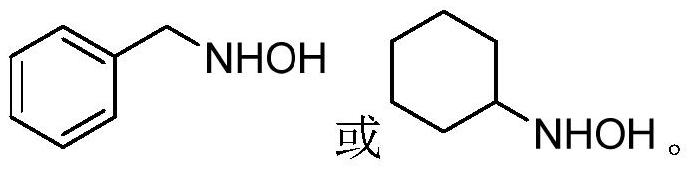
Synthesis method of polysubstituted isoxazolidine
A technology of isoxazolidine and synthesis method, which is applied in the field of biomedicine, can solve the problems of expensive, difficult to remove heavy metals, and limit the application of pharmaceutical industry, etc., and achieve the effect of cheap raw materials, good stereoselectivity of products, and wide application range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of (E)-2,5-dibenzyl-3-phenylisoxazolidine
[0034]
[0035] Phenylacetylene (102mg, 1.0mmol), acetophenone (132mg, 1.1mmol), potassium tert-butoxide (168mg, 1.5mmol), and dimethyl sulfoxide (5mL) were sequentially added to the reaction flask, and the temperature was raised to 100°C under stirring. , keep stirring for 40 minutes. Then add N-benzylhydroxylamine (135mg, 1.2mmol) into the reaction flask, keep warm at 100°C and react for 6 hours, TLC plate, the raw material point disappears, that is, the reaction is complete, stop stirring. Add 10 mL of distilled water, adjust the pH value to neutral with dilute hydrochloric acid, extract twice with ethyl acetate, wash the organic phase with saturated NaCl solution, dry over anhydrous magnesium sulfate, remove magnesium sulfate by filtration, and remove the solvent by rotary evaporation. The obtained oil and sodium cyanoborohydride (113 mg, 1.8 mmol) were dissolved in 5 mL of methanol, and a small amount of m...
Embodiment 2
[0037] Preparation of (E)-2-benzyl-5-p-tolylmethyl-3-phenylisoxazolidine
[0038]
[0039] Add p-methylphenylacetylene (116mg, 1.0mmol), acetophenone (132mg, 1.1mmol), sodium tert-butoxide (144mg, 1.5mmol), and dimethyl sulfoxide (5mL) into the reaction flask in turn, and heat up under stirring To 100°C, keep stirring for 40 minutes. Then add N-benzylhydroxylamine (135mg, 1.2mmol) into the reaction bottle, keep warm at 100°C and react for 5 hours, TLC plate, the raw material point disappears, that is, the reaction is complete, stop stirring. Add 10 mL of distilled water, adjust the pH value to neutral with dilute hydrochloric acid, extract twice with ethyl acetate, wash the organic phase with saturated NaCl solution, dry over anhydrous magnesium sulfate, remove magnesium sulfate by filtration, and remove the solvent by rotary evaporation. The obtained oil and sodium cyanoborohydride (113 mg, 1.8 mmol) were dissolved in 5 mL of methanol, and a small amount of methyl orange ...
Embodiment 3
[0041] Preparation of (E)-2-benzyl-5-p-nitrobenzyl-3-phenylisoxazolidine
[0042]
[0043] Add p-nitrophenylacetylene (147mg, 1.0mmol), acetophenone (132mg, 1.1mmol), sodium tert-butoxide (144mg, 1.5mmol), and dimethyl sulfoxide (5mL) into the reaction flask in turn, and heat up under stirring To 110°C, keep stirring for 30 minutes. Then add N-benzylhydroxylamine (135mg, 1.2mmol) into the reaction bottle, keep warm at 110°C and react for 5 hours, TLC plate, the raw material point disappears, that is, the reaction is complete, stop stirring. Add 10 mL of distilled water, adjust the pH value to neutral with dilute hydrochloric acid, extract twice with ethyl acetate, wash the organic phase with saturated NaCl solution, dry over anhydrous magnesium sulfate, remove magnesium sulfate by filtration, and remove the solvent by rotary evaporation. The obtained oil and sodium cyanoborohydride (113 mg, 1.8 mmol) were dissolved in 5 mL of methanol, and a small amount of methyl orange i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com