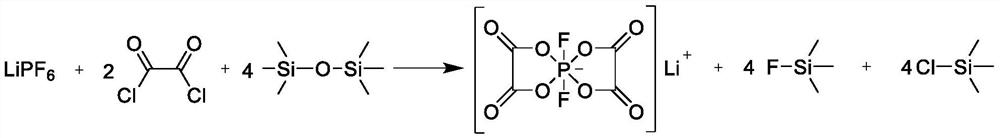
Lithium difluoro-bis(oxalate)phosphate as well as preparation method and application thereof
A technology of difluorobis-oxalate lithium phosphate and bis-oxalate lithium phosphate, which is applied in chemical instruments and methods, secondary batteries, electrochemical generators, etc. Low problems, to achieve the effect of ensuring purity and quality, avoiding complicated operations, and simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
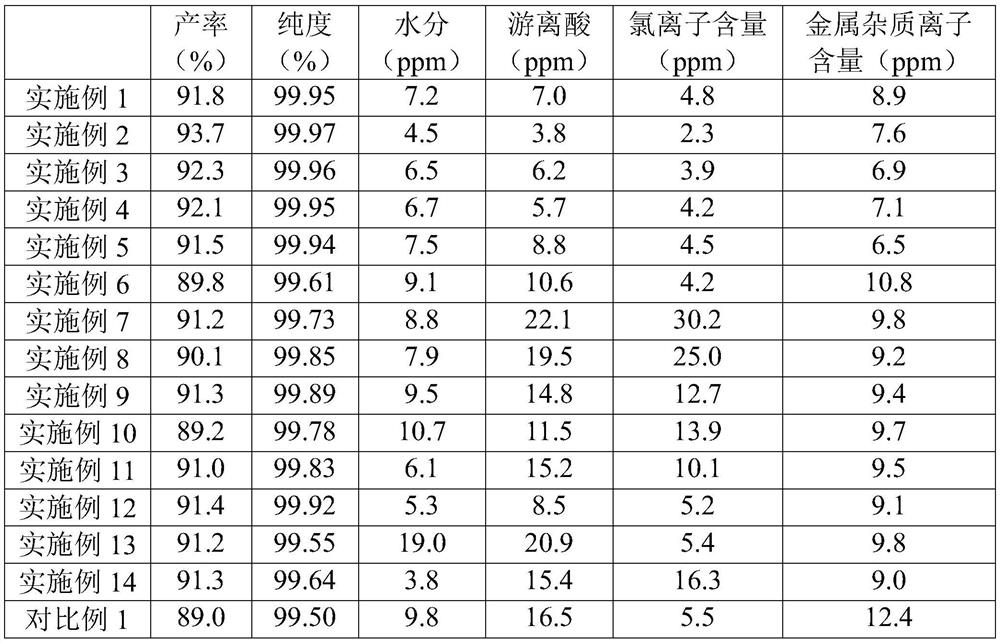
Examples
Embodiment 1
[0057] This embodiment provides a lithium difluorobisoxalate phosphate, the preparation method of the lithium difluorobisoxalate phosphate is as follows:
[0058] (1) In a glove box with a nitrogen atmosphere with a moisture content of less than 10ppm, add 250g of dimethyl carbonate dehydrated to 8ppm into a three-necked reaction flask, and simultaneously add 15.19g (0.1mol) of lithium hexafluorophosphate and 25.38g (0.2mol) of oxalyl chloride ), take the three-necked reaction bottle out of the glove box, place it on a constant temperature magnetic stirring device, heat it to 30°C, and add 64.95g (0.4mol) of hexamethyldisiloxane dropwise to the three-necked reaction via a constant pressure dropping funnel In the bottle, the molar ratio of lithium hexafluorophosphate to oxalyl chloride to hexamethyldisiloxane is 1:2:4, and the reaction is carried out with sufficient stirring, and the trimethylfluorosilane gas generated during the reaction is introduced into alkali such as potass...
Embodiment 2
[0061] This embodiment provides a lithium difluorobisoxalate phosphate, the preparation method of the lithium difluorobisoxalate phosphate is as follows:
[0062] (1) In a glove box with a nitrogen atmosphere with a moisture content of less than 10ppm, add 152g of diethyl carbonate dehydrated to 7ppm into a three-necked reaction flask, and simultaneously add 15.19g (0.1mol) of lithium hexafluorophosphate and 30.46g (0.24mol) of oxalyl chloride ), take the three-necked reaction bottle out of the glove box, place it on a constant temperature magnetic stirring device, heat it to 60°C, and add 73.07g (0.45mol) of hexamethyldisiloxane dropwise to the three-necked reaction via a constant pressure dropping funnel In the bottle, the molar ratio of lithium hexafluorophosphate, oxalyl chloride, and hexamethyldisiloxane is 1:2.4:4.5, fully stirred for reaction, and the trimethylfluorosilane gas generated during the reaction is introduced into alkali such as potassium hydroxide solution A...
Embodiment 3
[0065] This embodiment provides a lithium difluorobisoxalate phosphate, the preparation method of the lithium difluorobisoxalate phosphate is as follows:
[0066] (1) In a glove box with a nitrogen atmosphere with a moisture content of less than 10ppm, add 300g of ethyl methyl carbonate dehydrated to 6ppm into a three-necked reaction flask, and simultaneously add 15.19g (0.1mol) of lithium hexafluorophosphate and 27.92g (0.22mol) of oxalyl chloride ), take the three-necked reaction bottle out of the glove box, place it on a constant temperature magnetic stirring device, heat it to 40°C, and add 68.20 g (0.42 mol) of hexamethyldisiloxane dropwise to the three-necked reaction via a constant pressure dropping funnel In the bottle, the molar ratio of lithium hexafluorophosphate, oxalyl chloride, and hexamethyldisiloxane is 1:2.2:4.2, fully stirred for reaction, and the trimethylfluorosilane gas generated during the reaction is introduced into alkali such as potassium hydroxide solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com