A kind of detection method of hydrazine hydrate in medicine
A technology of hydrazine hydrate and drugs, which is applied in the field of detection of hydrazine hydrate in drugs, can solve problems such as accuracy and stability to be improved, and achieve the effects of good applicability and reliability, improved separation, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Take 0.5g of p-dimethylaminobenzaldehyde, accurately weigh it, put it in a 25ml volumetric flask, add glacial acetic acid-ethanol (1:9) solution to dissolve to constant volume, and obtain a derivative reagent;
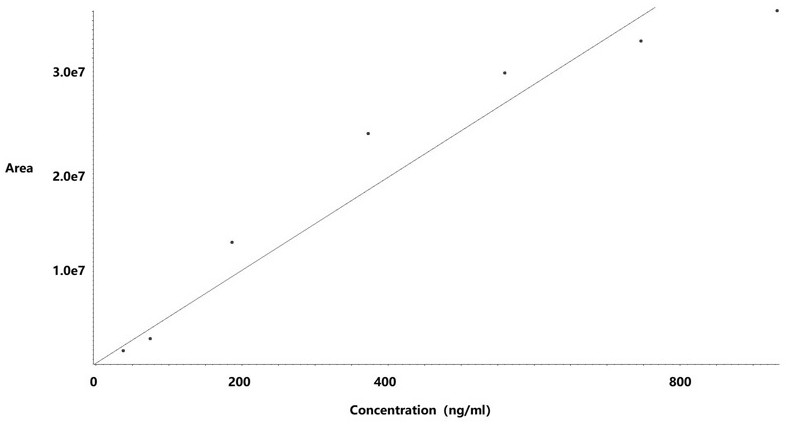
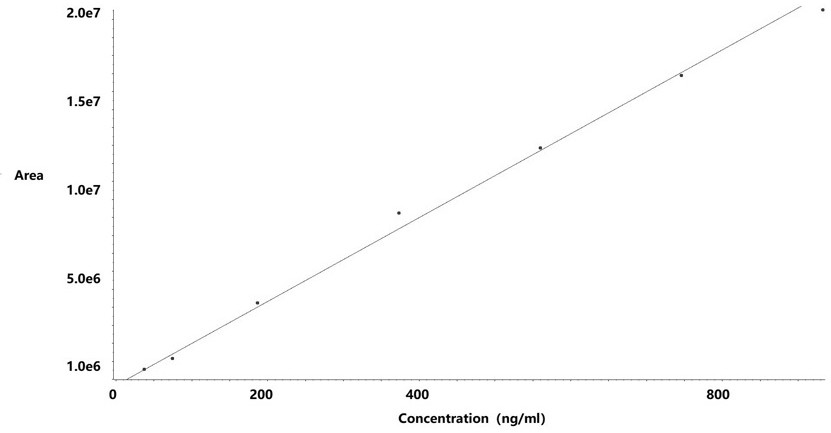
[0042] (2) Take an appropriate amount of hydrazine standard solution in a 10ml volumetric flask, add water to dilute to the mark, shake well, and dilute with ethanol to prepare standards with hydrazine hydrate concentrations of 18.66~932.81 ng / ml (hydrazine hydrate limit percentage: 5~250%) curve solution;
[0043] (3) Take 50 mg of raw drug powder (oseltamivir) and add ethanol to dissolve it, use a 10 ml volumetric flask to obtain the test solution, take 50 mg of raw drug powder and add it to the reference solution (373.13 ng / mL) bottle to constant volume to obtain the spiked solution;
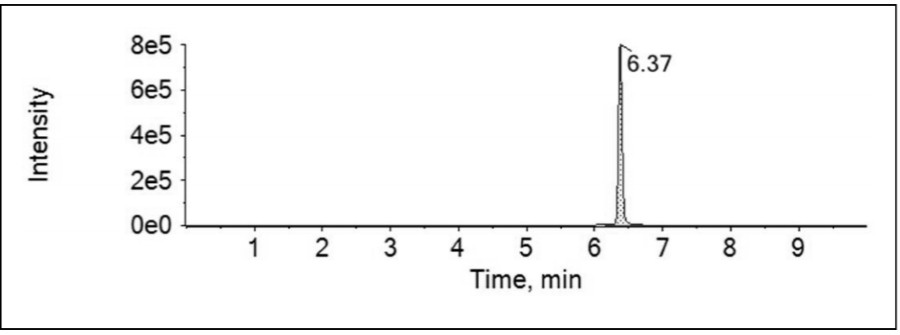
[0044] (4) Take ethanol solvent, standard curve solution, test solution and spiked solution respectively and add derivatization reagents for derivatization reaction, sonic...
Embodiment 2
[0076] Referring to the detection method of Example 1, the volume ratio is 1:19, the only difference is to adjust the temperature and time of the derivatization reaction, detect the peak area of the 100% limit reference solution (373.13ng / mL), and repeat the measurement 3 times to get the average value. The experimental parameters and results are shown in Table 5 below.
[0077] The results show that the derivatization can be completed when the derivatization temperature is 25℃ and the derivatization time is 40~60min, especially when the sonication is 40min, the derivatization efficiency is the highest and the derivative product is the most stable. However, when the derivatization temperature was 50°C, the derivative products in the system were unstable, and the peak area dropped sharply, which may have introduced interfering substances to affect the measurement results.
[0078] table 5
[0079]
Embodiment 3
[0081] Referring to the detection method of Example 1, the volume ratio is 1:19, the only difference is that the solvent of the derivatization reagent is adjusted, the recovery rate of the spiked solution is detected, and the measurement is repeated 6 times. The experimental parameters and results are shown in Table 6 below.
[0082] The results showed that the use of methanol as a solvent for the derivatization reagent would cause great interference to the system, and the use of aqueous solution would affect the solubility of the test sample, which were not suitable for the detection of hydrazine hydrate in drugs. In the glacial acetic acid-ethanol (1:9) system, the recovery rate of the spiked solution was the highest and the stability was the strongest, meeting the detection requirements.
[0083] Table 6
[0084]
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com