Method of treating neutrophilic conditions
A neutrophil and neutrophil technology, applied in the field of treatment of neutrophilic diseases, can solve the problems of patient discomfort, leukocyte apheresis, time-consuming and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
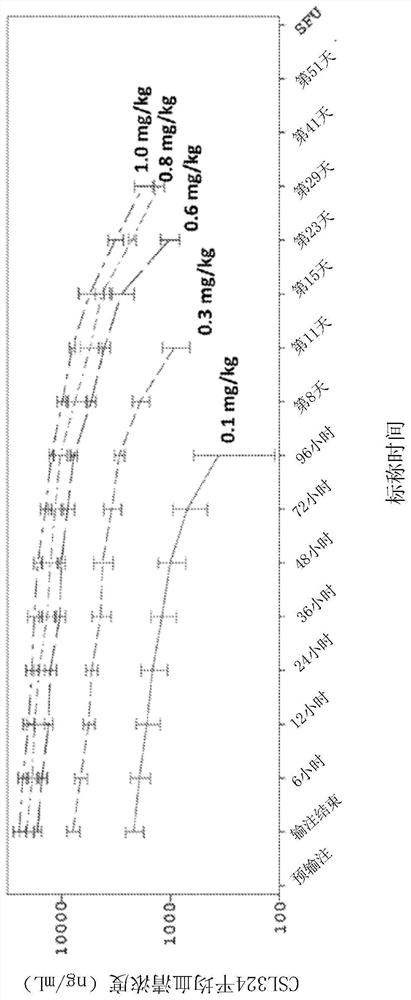
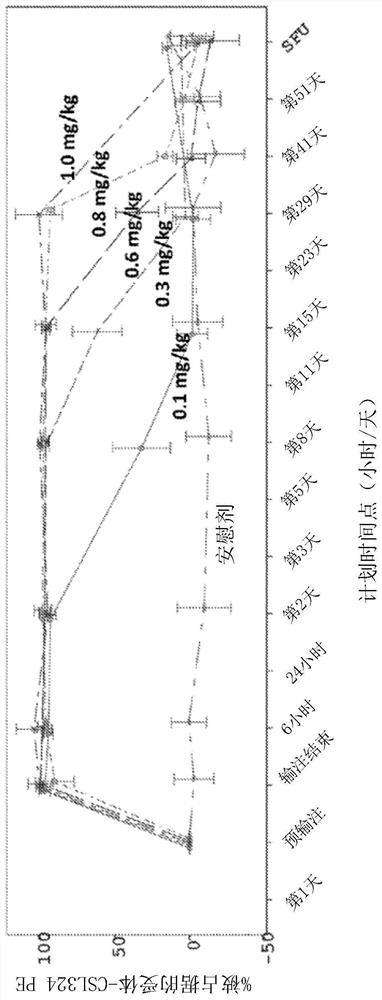
[0373] Example 1 - Safety, Pharmacokinetics (PK) and Pharmacodynamics (PD) of Administering G-CSFR-binding Antibody C1.2G to Healthy Adult Subjects
[0374] A Phase 1 clinical trial was conducted to evaluate the safety of single ascending doses (Parts A and B) and repeated (Part C) intravenous (IV) infusions of CSL324 (also referred to herein as C1.2G) in healthy subjects and tolerance.
[0375] method
[0376] This trial is the first single-center randomized double-blind placebo-controlled study in humans to evaluate the safety, tolerability, PK and pharmacodynamics (PD) of single ascending doses and repeated doses of IV CSL324 in healthy human subjects ). The study consisted of 3 parts: Parts A, B and C. Routine blinded review of safety, tolerability, PK and selected PD data was conducted by a Safety Review Committee (SRC) to guide dose selection. The trial is described in the Australia New Zealand Clinical Trials Register (ANZCTR), accession number ACTRN12616000846426...
Embodiment 2
[0468] Example 2 - Treatment of neutrophilic dermatosis with G-CSFR binding antibody CSL324
[0469] Research design
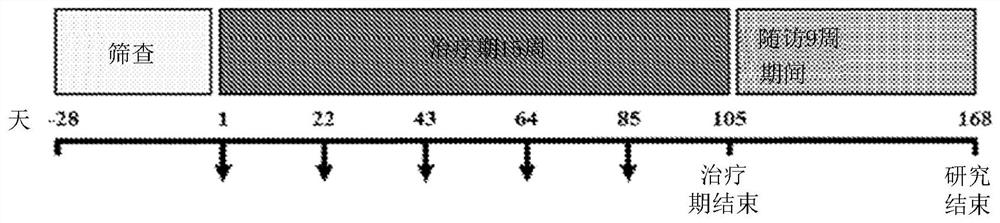
[0470] The safety and PK of repeated doses of intravenously administered CSL324 in subjects with HS and PPP were investigated using a multicentre, open-label, two-protocol repeat-dose study. This study also investigated the preliminary efficacy of CSL324 in subjects with HS and PPP.
[0471] The study included a 28-day screening period, a 15-week treatment period and a 9-week follow-up period. The study had 2 cohorts. Each cohort consisted of 20 subjects with HS (n=10) or PPP (n=10) ( image 3 ). CSL324 was initially administered to subjects participating in Cohort #1 as a 60-minute IV infusion of 0.3 mg / kg CSL324 at 21-day intervals on Days 1, 22, 43, 64, and 85. When the first 5 subjects in Cohort #1 administered CSL324 completed the 15-week treatment period, subjects in Cohort #2 were administered CSL324. The safest CSL324 dose (≥0.1 and ≤0.6 mg / kg)...
Embodiment 3
[0545] Example 3 - CSL324 reduces neutrophil migration associated with CXCR1 expression, a marker that is upregulated in HS patients.
[0546] CXCR1 expression in HS patients
[0547] Neutrophils on the surface of neutrophils were assessed by flow cytometry using whole blood samples from patients with hidradenitis suppurativa (HS; n=15) compared with neutrophils from whole blood samples from age- and sex-matched healthy controls. Levels of the cell migration marker CXCR1 (defined by high side scatter (SSC), CD11b+CD49-).
[0548] Such as Figure 7 As shown in A, CXCR1 expression was significantly higher in neutrophils from HS patient samples compared to healthy controls (n=15). A further analysis was observed showing the correlation between abscesses in HS patients and nodule counts, disease activity assessment forms, and CXCR1 expression on neutrophils in HS patients (n=14) ( Figure 7 B; r 2 = 0.3532, p = 0.0250).
[0549] CSL324 reduces G-CSF-induced expression of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com