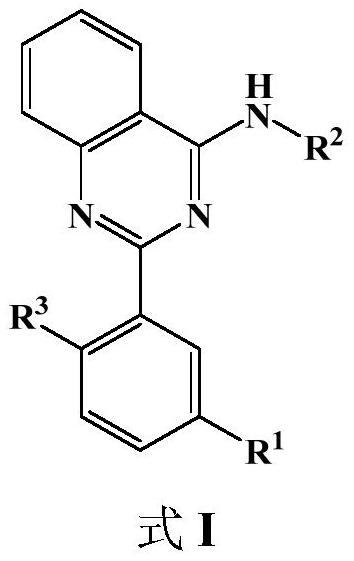
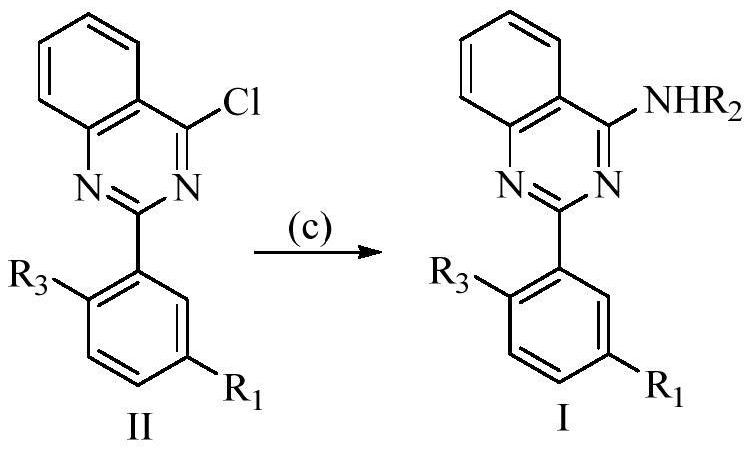
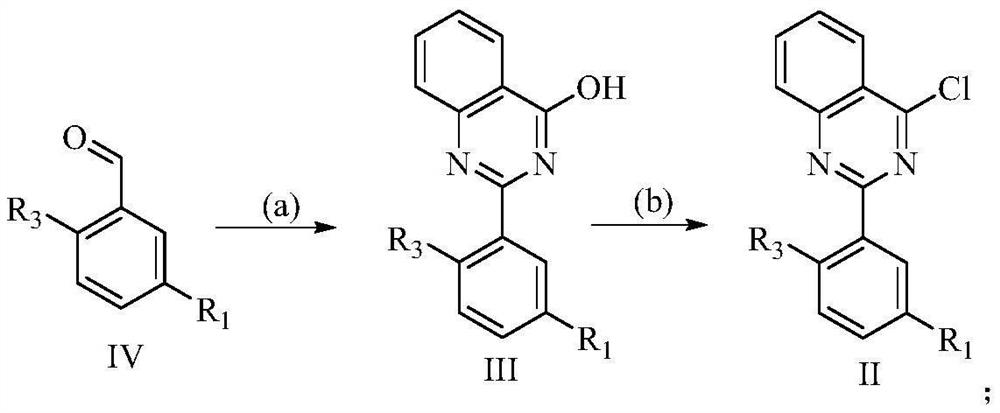
2-arylamine-4-amido quinazoline compound as well as preparation method and application thereof
A compound, quinazoline technology, used in the field of medicine for diseases and disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: Preparation 3-(4-(ethylamino) quinazoline-2-yl)-4-methoxybenzonitrile (I-1)
[0098] Step A: Preparation of 5-cyano-2-methoxybenzaldehyde
[0099] Add DMF (300mL) to a 500ml reaction flask equipped with a thermometer and a drying tube, turn on mechanical stirring, add 5-bromo-2-methoxybenzaldehyde (20g, 93mmol), stir for 10min to dissolve, and then add cyanide Cuprous copper (16.7g, 186mmol), under the protection of nitrogen, stirred and heated to 145-150°C for micro-reflux reaction, the reaction solution gradually dissolved, and then the reaction solution gradually became dark green viscous, reacted for 14-16h, TLC (petroleum Ether / ethyl acetate=4 / 1) the reaction is completed, the reaction solution is cooled to room temperature, and the copper salt is removed by filtration, and the filtrate is slowly added to (800 mL) cold water under stirring, and a brown-green viscous solid is precipitated, and the solid is respectively washed with ammonia solution ( 2...
Embodiment 2
[0106] Embodiment 2: Preparation of 3-(4-(benzylamino)quinazolin-2-yl)-4-methoxybenzonitrile (I-2)
[0107] The procedure is the same as in Example 1, using benzylamine as the reactant in step D.
[0108] Pale yellow solid, yield 94.5%, m.p.187-190℃; 1 H NMR (400MHz, DMSO-d 6 )δ:3.80(s,3H),4.78(d,J=6.0Hz,2H),7.25-7.58(m,4H),7.40-7.42(m,2H),7.55-7.56(m,1H),7.72 -7.90(m,4H),8.33(d,J=7.6Hz,1H),8.95(s,1H,adding D 2 O disappear); 13 CNMR (100MHz, DMSO-d 6 )δ:44.18,56.54,102.79,113.67,113.90,119.45,123.07,126.36,127.25,127.97,128.19,128.71,131.54,133.26,134.86,134.88,140.12,150.13,159.87,160.11,161.18;HRMS(ESITOF)m / z:Calcd.for C 23 h 18 N 4 O[M+H] + 367.1559,found 367.1555.
Embodiment 3
[0109] Example 3: Preparation of 4-methoxy-3-(4-((pyridin-3-ylmethyl)amino)quinazolin-2-yl)benzonitrile (I-3)
[0110] The operation is the same as in Example 1, using 3-aminomethylpyridine as the reactant in step D.
[0111] Pale yellow solid, yield 80.25%, m.p.189-192℃(dec); 1 H NMR (400MHz, DMSO-d 6 )δ:3.81(s,3H),4.78(d,J=5.6Hz,2H),7.29-7.31(m,1H),7.35-7.38(m,1H),7.55-7.59(m,1H),7.74 -7.91(m,5H),8.30(d,J=8.0Hz,1H),8.47(d,J=0.8Hz,1H),8.64(s,1H).8.98-8.99(m,1H,adding D 2 O disappear); 13 C NMR (100MHz, DMSO-d 6 )δ:41.99,56.58,102.85,113.67,113.87,123.04,123.89,126.45,128.23,133.34,134.78,134.93,135.54,135.84,148.57,149.68,159.80,160.11,161.19;HRMS(ESITOF)m / z:Calcd .forC 22 h 17 N 5 O[M+H] + 368.1511,found 368.1507.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com